
Troglitazone is an antidiabetic and anti-inflammatory drug, and a member of the drug class of the thiazolidinediones. It was prescribed for people with diabetes mellitus type 2.

Exelixis, Inc. is a genomics-based drug discovery company located in Alameda, California, and the producer of Cometriq, a treatment approved by the U.S. Food and Drug Administration (FDA) for medullary thyroid cancer with clinical activity in several other types of metastatic cancer.

Daiichi Sankyo Company, Limited is a global pharmaceutical company and the second-largest pharmaceutical company in Japan. It achieved JPY 981.8 billion in revenue in 2019. The company owns the American biotechnology company Plexxikon, American pharmaceutical company American Regent, German biotechnology company U3 Pharma, and recently sold Ranbaxy Laboratories in India. Daiichi Sankyo Co., Ltd. is the producer of Benicar (Olmesartan), an angiotensin II receptor antagonist and top selling drug in the U.S. Global sales of Olmesartan in 2013 were 300.2 billion yen.
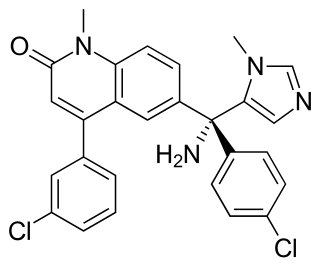
Tipifarnib is a farnesyltransferase inhibitor. Farnesyltransferase inhibitors block the activity of the farnesyltransferase enzyme by inhibiting prenylation of the CAAX tail motif, which ultimately prevents Ras from binding to the membrane, rendering it inactive.
Nimotuzumab is a humanized monoclonal antibody that as of 2014 had orphan status in the US and EU for glioma, and marketing approval in India, China, and other countries for squamous cell carcinomas of the head and neck, and was undergoing several clinical trials.

Lestaurtinib is a tyrosine kinase inhibitor structurally related to staurosporine. This semisynthetic derivative of the indolocarbazole K252a was investigated by Cephalon as a treatment for various types of cancer. It is an inhibitor of the kinases fms-like tyrosine kinase 3 (FLT3), Janus kinase 2 (JAK2), tropomyosin receptor kinase (trk) A (TrkA), TrkB and TrkC.

Sapacitabine is a chemotherapeutic drug developed by US biotechnology firm Cyclacel currently undergoing clinical trials against leukemia.
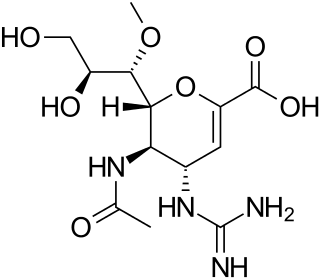
Laninamivir (CS-8958) is a neuraminidase inhibitor that is a drug used for the treatment and prophylaxis of Influenzavirus A and Influenzavirus B. It is currently in Phase III clinical trials. It is a long-acting neuraminidase inhibitor administered by nasal inhalation.

Genta Incorporated was a biopharmaceutical company started in La Jolla, California, which discovered and developed innovative drugs for the treatment of patients with cancer. Founded in 1989 by a highly skilled entrepreneur, the company focused on a novel technology known as antisense, which targets gene products that are associated with the onset and progression of serious diseases. At that time, only Ionis Pharmaceuticals, Inc. was conducting significant research with this technology. Antisense is a short span of oligonucleotides – modified DNA structures ranging from about 12-24 bases that selectively bind to specific RNA. The intent is to block expression of an aberrant protein that contributes to the disease of interest. Genta in-licensed three different antisense molecules that blocked Bcl-2, a fibroblast growth factor (FGF), and the gene c-myb, respectively.

Melior Discovery, Inc. is a private biopharmaceutical company based in Exton, Pennsylvania, USA. The company specializes in drug repositioning and has established a proprietary phenotypic screening platform that it uses for this purpose. Melior also offers certain contract research organization (CRO) services comprising animal models representing different disease conditions. The Company has issued press releases disclosing partnerships with Pfizer, Merck & Co., Johnson & Johnson, and AstraZeneca, all citing the use of the company’s drug repositioning technology. Melior Discovery has also used its technology to discover its own drug candidates.

Vemurafenib (INN), sold under the brand name Zelboraf, is a medication used for the treatment of late-stage melanoma. It is an inhibitor of the B-Raf enzyme and was developed by Plexxikon.

ALK inhibitors are anti-cancer drugs that act on tumours with variations of anaplastic lymphoma kinase (ALK) such as an EML4-ALK translocation. They fall under the category of tyrosine kinase inhibitors, which work by inhibiting proteins involved in the abnormal growth of tumour cells. All the current approved ALK inhibitors function by binding to the ATP pocket of the abnormal ALK protein, blocking its access to energy and deactivating it. A majority of ALK-rearranged NSCLC harbour the EML4-ALK fusion, although as of 2020, over 92 fusion partners have been discovered in ALK+ NSCLC. For each fusion partner, there can be several fusion variants depending on the position the two genes were fused at, and this may have implications on the response of the tumour and prognosis of the patient.
Santaris Pharma A/S was a biopharmaceutical company founded in 2003 in Copenhagen, Denmark. The company also had a branch in San Diego, California that opened in 2009. Created by a merger between Cureon and Pantheco, Santaris developed RNA-targeted medicines using a Locked Nucleic Acid (LNA) Drug Platform and Drug Development Engine.

Teneligliptin is a pharmaceutical drug for the treatment of type 2 diabetes mellitus. It belongs to the class of anti-diabetic drugs known as dipeptidyl peptidase-4 inhibitors or "gliptins".
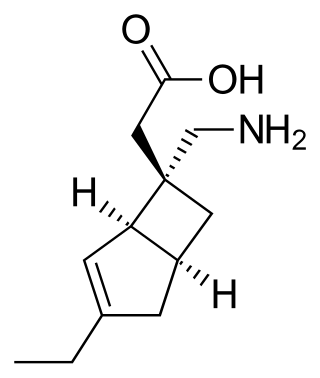
Mirogabalin is a medication developed by Daiichi Sankyo, a gabapentinoid. Gabapentin and pregabalin are also members of this class. As a gabapentinoid, mirogabalin binds to the α2δ subunit of voltage-gated calcium channel, but with significantly higher potency than pregabalin. It has shown promising results in Phase II clinical trials for the treatment of diabetic peripheral neuropathic pain.

PD-1 inhibitors and PD-L1 inhibitors are a group of checkpoint inhibitor anticancer drugs that block the activity of PD-1 and PDL1 immune checkpoint proteins present on the surface of cells. Immune checkpoint inhibitors are emerging as a front-line treatment for several types of cancer.
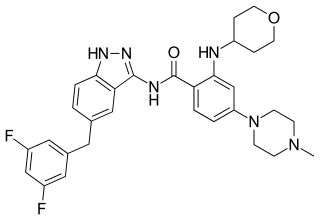
Entrectinib, sold under the brand name Rozlytrek, is an anti-cancer medication used to treat ROS1-positive non-small cell lung cancer and NTRK fusion-positive solid tumors. It is a selective tyrosine kinase inhibitor (TKI), of the tropomyosin receptor kinases (TRK) A, B and C, C-ros oncogene 1 (ROS1) and anaplastic lymphoma kinase (ALK).

Larotrectinib, sold under the brand name Vitrakvi, is a medication for the treatment of cancer. It is an inhibitor of tropomyosin kinase receptors TrkA, TrkB, and TrkC. It was discovered by Array BioPharma and licensed to Loxo Oncology in 2013.

Gedatolisib (PF-05212384) is an experimental drug for treatment of cancer in development by Celcuity, Inc. The mechanism of action is accomplished by binding the different p110 catalytic subunit isoforms of PI3K and the kinase site of mTOR.

Ultragenyx is an American biopharmaceutical company involved in the research and development of novel products for treatment of rare and ultra-rare genetic diseases for which there are typically no approved treatments and high unmet medical need. The company works with multiple drug modalities including biologics, small molecule, gene therapies, and ASO and mRNAs in the disease categories of bone, endocrine, metabolic, muscle and CNS diseases.

















