
Solder is a fusible metal alloy used to create a permanent bond between metal workpieces. Solder is melted in order to wet the parts of the joint, where it adheres to and connects the pieces after cooling. Metals or alloys suitable for use as solder should have a lower melting point than the pieces to be joined. The solder should also be resistant to oxidative and corrosive effects that would degrade the joint over time. Solder used in making electrical connections also needs to have favorable electrical characteristics.

Plumbing is any system that conveys fluids for a wide range of applications. Plumbing uses pipes, valves, plumbing fixtures, tanks, and other apparatuses to convey fluids. Heating and cooling (HVAC), waste removal, and potable water delivery are among the most common uses for plumbing, but it is not limited to these applications. The word derives from the Latin for lead, plumbum, as the first effective pipes used in the Roman era were lead pipes.

In physiology, dehydration is a lack of total body water, with an accompanying disruption of metabolic processes. It occurs when free water loss exceeds free water intake, usually due to exercise, disease, or high environmental temperature. Mild dehydration can also be caused by immersion diuresis, which may increase risk of decompression sickness in divers.
Countercurrent exchange is a mechanism occurring in nature and mimicked in industry and engineering, in which there is a crossover of some property, usually heat or some chemical, between two flowing bodies flowing in opposite directions to each other. The flowing bodies can be liquids, gases, or even solid powders, or any combination of those. For example, in a distillation column, the vapors bubble up through the downward flowing liquid while exchanging both heat and mass.
Water purification is the process of removing undesirable chemicals, biological contaminants, suspended solids, and gases from water. The goal is to produce water that is fit for specific purposes. Most water is purified and disinfected for human consumption, but water purification may also be carried out for a variety of other purposes, including medical, pharmacological, chemical, and industrial applications. The history of water purification includes a wide variety of methods. The methods used include physical processes such as filtration, sedimentation, and distillation; biological processes such as slow sand filters or biologically active carbon; chemical processes such as flocculation and chlorination; and the use of electromagnetic radiation such as ultraviolet light.

Tap water is water supplied through a tap, a water dispenser valve. In many countries, tap water usually has the quality of drinking water. Tap water is commonly used for drinking, cooking, washing, and toilet flushing. Indoor tap water is distributed through indoor plumbing, which has existed since antiquity but was available to very few people until the second half of the 19th century when it began to spread in popularity in what are now developed countries. Tap water became common in many regions during the 20th century, and is now lacking mainly among people in poverty, especially in developing countries.
Diuresis is the excretion of urine, especially when excessive (polyuria). The term collectively denotes the physiologic processes underpinning increased urine production by the kidneys during maintenance of fluid balance.

Hard water is water that has a high mineral content. Hard water is formed when water percolates through deposits of limestone, chalk or gypsum, which are largely made up of calcium and magnesium carbonates, bicarbonates and sulfates.

In metallurgy, a flux is a chemical reducing agent, flowing agent, or purifying agent. Fluxes may have more than one function at a time. They are used in both extractive metallurgy and metal joining.

Saline is a mixture of sodium chloride (salt) and water. It has a number of uses in medicine including cleaning wounds, removal and storage of contact lenses, and help with dry eyes. By injection into a vein, it is used to treat hypovolemia such as that from gastroenteritis and diabetic ketoacidosis. Large amounts may result in fluid overload, swelling, acidosis, and high blood sodium. In those with long-standing low blood sodium, excessive use may result in osmotic demyelination syndrome.

Hydronics is the use of liquid water or gaseous water (steam) or a water solution as a heat-transfer medium in heating and cooling systems. The name differentiates such systems from oil and refrigerant systems.
A corrosion inhibitor or anti-corrosive is a chemical compound added to a liquid or gas to decrease the corrosion rate of a metal that comes into contact with the fluid. The effectiveness of a corrosion inhibitor depends on fluid composition and dynamics. Corrosion inhibitors are common in industry, and also found in over-the-counter products, typically in spray form in combination with a lubricant and sometimes a penetrating oil. They may be added to water to prevent leaching of lead or copper from pipes.

A pipe is a tubular section or hollow cylinder, usually but not necessarily of circular cross-section, used mainly to convey substances which can flow — liquids and gases (fluids), slurries, powders and masses of small solids. It can also be used for structural applications; a hollow pipe is far stiffer per unit weight than the solid members.

The Lead and Copper Rule (LCR) is a United States federal regulation that limits the concentration of lead and copper allowed in public drinking water at the consumer's tap, as well as limiting the permissible amount of pipe corrosion occurring due to the water itself. The U.S. Environmental Protection Agency (EPA) first issued the rule in 1991 pursuant to the Safe Drinking Water Act (SDWA). The EPA promulgated the regulations following studies that concluded that copper and lead have an adverse effect on individuals. The LCR limits the levels of these metals in water through improving water treatment centers, determining copper and lead levels for customers who use lead plumbing parts, and eliminating the water source as a source of lead and copper. If the lead and copper levels exceed the "action levels", water suppliers are required to educate their consumers on how to reduce exposure to lead. In a 2005 report EPA stated that the LCR requirements had been effective in 96 percent of systems serving at least 3,300 people.

A fitting or adapter is used in pipe systems to connect sections of pipe or tube, adapt to different sizes or shapes, and for other purposes such as regulating fluid flow. These fittings are used in plumbing to manipulate the conveyance of fluids such as water for potatory, irrigational, sanitary, and refrigerative purposes, gas, petroleum, liquid waste, or any other liquid or gaseous substances required in domestic or commercial environments, within a system of pipes or tubes, connected by various methods, as dictated by the material of which these are made, the material being conveyed, and the particular environmental context in which they will be used, such as soldering, mortaring, caulking, plastic welding, welding, friction fittings, threaded fittings, and compression fittings.
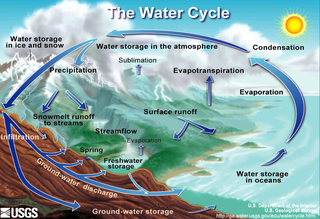
Freshwater environmental quality parameters are those chemical, physical and biological parameters that can be used to characterise a freshwater body. Because almost all water bodies are dynamic in their composition, the relevant quality parameters are typically expressed as a range of expected concentrations.

Soldering is a process of joining two metal surfaces together using a filler metal called solder. The soldering process involves heating the surfaces to be joined and melting the solder, which is then allowed to cool and solidify, creating a strong and durable joint.

Copper tubing is available in two basic types of tube—plumbing tube and air conditioning/refrigeration (ACR) tube, and in both drawn (hard) and annealed (soft) tempers. Because of its high level of corrosion resistance, it is used for water distribution systems, oil fuel transfer lines, non-flammable medical-gas systems, and as a refrigerant line in HVAC systems. Copper tubing is joined using flare connection, compression connection, pressed connection, or solder.

Water chlorination is the process of adding chlorine or chlorine compounds such as sodium hypochlorite to water. This method is used to kill bacteria, viruses and other microbes in water. In particular, chlorination is used to prevent the spread of waterborne diseases such as cholera, dysentery, and typhoid.

A lead service line is a pipe made of lead which is used in potable water distribution to connect a water main to a user's premises.















