
A pneumatic trough is a piece of laboratory apparatus used for collecting gases, such as hydrogen, oxygen and nitrogen. It is mainly made of glass or various fibres and are of various sizes. It was invented by Stephen Hales. [1]

A pneumatic trough is a piece of laboratory apparatus used for collecting gases, such as hydrogen, oxygen and nitrogen. It is mainly made of glass or various fibres and are of various sizes. It was invented by Stephen Hales. [1]
Four items are required for gas collection with a pneumatic trough: [2]
Pneumatic troughs require a liquid such as water. Scientists also have used mercury in pneumatic troughs, but usually only for the collection of water-soluble gases. Health and safety issues surrounding mercury generally prohibit its use in modern-day pneumatic troughs. [3]

The bottle is filled with water, inverted, and placed into the pneumatic trough already containing water. The outlet tube from the gas-generating apparatus is inserted into the opening of the bottle so that gas can bubble up through it, displacing the water within. [4]

A thermometer is a device that measures temperature or temperature gradient. A thermometer has two important elements: (1) a temperature sensor in which some change occurs with a change in temperature; and (2) some means of converting this change into a numerical value. Thermometers are widely used in technology and industry to monitor processes, in meteorology, in medicine, and in scientific research.

Laboratory glassware is a variety of equipment used in scientific work, traditionally made of glass. Glass may be blown, bent, cut, molded, or formed into many sizes and shapes. It is commonly used in chemistry, biology, and analytical laboratories. Many laboratories have training programs to demonstrate how glassware is used and to alert first–time users to the safety hazards involved with using glassware.

An incandescent light bulb, incandescent lamp or incandescent light globe is an electric light with a filament that is heated until it glows. The filament is enclosed in a glass bulb that is either evacuated or filled with inert gas to protect the filament from oxidation. Electric current is supplied to the filament by terminals or wires embedded in the glass. A bulb socket provides mechanical support and electrical connections.

A pipette is a type of laboratory tool commonly used in chemistry and biology to transport a measured volume of liquid, often as a media dispenser. Pipettes come in several designs for various purposes with differing levels of accuracy and precision, from single piece glass pipettes to more complex adjustable or electronic pipettes. Many pipette types work by creating a partial vacuum above the liquid-holding chamber and selectively releasing this vacuum to draw up and dispense liquid. Measurement accuracy varies greatly depending on the instrument.

The mercury-in-glass or mercury thermometer is a thermometer that uses the thermal expansion and contraction of liquid mercury to indicate the temperature.

Carbonated water is water containing dissolved carbon dioxide gas, either artificially injected under pressure, or occurring due to natural geological processes. Carbonation causes small bubbles to form, giving the water an effervescent quality. Common forms include sparkling natural mineral water, club soda, and commercially produced sparkling water.

In the signage industry, neon signs are electric signs lighted by long luminous gas-discharge tubes that contain rarefied neon or other gases. They are the most common use for neon lighting, which was first demonstrated in a modern form in December 1910 by Georges Claude at the Paris Motor Show. While they are used worldwide, neon signs were popular in the United States from about the 1920s to 1950s. The installations in Times Square, many originally designed by Douglas Leigh, were famed, and there were nearly 2,000 small shops producing neon signs by 1940. In addition to signage, neon lighting is used frequently by artists and architects, and in plasma display panels and televisions. The signage industry has declined in the past several decades, and cities are now concerned with preserving and restoring their antique neon signs.

Stephen Hales was an English clergyman who made major contributions to a range of scientific fields including botany, pneumatic chemistry and physiology. He was the first person to measure blood pressure. He also invented several devices, including a ventilator, a pneumatic trough and a surgical forceps for the removal of bladder stones. In addition to these achievements, he was a philanthropist and wrote a popular tract on alcoholic intemperance.
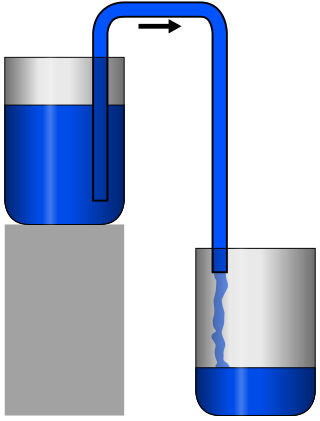
A siphon is any of a wide variety of devices that involve the flow of liquids through tubes. In a narrower sense, the word refers particularly to a tube in an inverted "U" shape, which causes a liquid to flow upward, above the surface of a reservoir, with no pump, but powered by the fall of the liquid as it flows down the tube under the pull of gravity, then discharging at a level lower than the surface of the reservoir from which it came.

A gas-filled tube, also commonly known as a discharge tube or formerly as a Plücker tube, is an arrangement of electrodes in a gas within an insulating, temperature-resistant envelope. Gas-filled tubes exploit phenomena related to electric discharge in gases, and operate by ionizing the gas with an applied voltage sufficient to cause electrical conduction by the underlying phenomena of the Townsend discharge. A gas-discharge lamp is an electric light using a gas-filled tube; these include fluorescent lamps, metal-halide lamps, sodium-vapor lamps, and neon lights. Specialized gas-filled tubes such as krytrons, thyratrons, and ignitrons are used as switching devices in electric devices.

In medicine, a nebulizer or nebuliser is a drug delivery device used to administer medication in the form of a mist inhaled into the lungs. Nebulizers are commonly used for the treatment of asthma, cystic fibrosis, COPD and other respiratory diseases or disorders. They use oxygen, compressed air or ultrasonic power to break up solutions and suspensions into small aerosol droplets that are inhaled from the mouthpiece of the device. An aerosol is a mixture of gas and solid or liquid particles.

A mercury-vapor lamp is a gas-discharge lamp that uses an electric arc through vaporized mercury to produce light. The arc discharge is generally confined to a small fused quartz arc tube mounted within a larger soda lime or borosilicate glass bulb. The outer bulb may be clear or coated with a phosphor; in either case, the outer bulb provides thermal insulation, protection from the ultraviolet radiation the light produces, and a convenient mounting for the fused quartz arc tube.

A eudiometer is a laboratory device that measures the change in volume of a gas mixture following a physical or chemical change.

A mercury-arc valve or mercury-vapor rectifier or (UK) mercury-arc rectifier is a type of electrical rectifier used for converting high-voltage or high-current alternating current (AC) into direct current (DC). It is a type of cold cathode gas-filled tube, but is unusual in that the cathode, instead of being solid, is made from a pool of liquid mercury and is therefore self-restoring. As a result mercury-arc valves, when used as intended, are far more robust and durable and can carry much higher currents than most other types of gas discharge tube. Some examples have been in continuous service, rectifying 50-ampere currents, for decades.

Drinking birds, also known as dunking birds, drinky birds, water birds, or dipping birds are toy heat engines that mimic the motions of a bird drinking from a water source. They are sometimes incorrectly considered examples of a perpetual motion device.

The Victor Meyer apparatus is the standard laboratory method for determining the molecular weight of a volatile liquid. It was developed by Viktor Meyer, who spelled his name Victor in publications at the time of its development. In this method, a known mass of a volatile solid or liquid under examination is converted into its vapour form by heating in a Victor Meyer's tube. The vapour displaces its own volume of air. The volume of air displaced at experimental temperature and pressure is calculated. Then volume of air displaced at standard temperature and pressure is calculated. Using this, mass of air displaced at 2.24 × 10−2 m3 of vapour at STP is calculated. This value represents the molecular mass of the substance. The apparatus consists of an inner Victor Meyer's tube, lower end of which is in form of a bulb. The upper end of tube has a side tube that leads to a trough filled with water. The Victor Meyer's tube is surrounded by an outer jacket. In the outer jacket, a liquid is placed, which boils at a temperature at least 30 K higher than the substance under examination. A small quantity of glass-wool or asbestos pad covers the lower end of the Victor Meyer's tube to prevent breakage, when a glass bottle containing the substance under examination is dropped to it

A gas bubbler is a piece of laboratory glassware which consists of a glass bulb filled with a small amount of fluid—usually mineral or silicone oil, less commonly mercury. The inlet to the bulb is connected to a ground glass joint, while the outlet is vented to the air.

In chemistry, a condenser is laboratory apparatus used to condense vapors – that is, turn them into liquids – by cooling them down.

In the history of science, pneumatic chemistry is an area of scientific research of the seventeenth, eighteenth, and early nineteenth centuries. Important goals of this work were the understanding of the physical properties of gases and how they relate to chemical reactions and, ultimately, the composition of matter. The rise of phlogiston theory, and its replacement by a new theory after the discovery of oxygen as a gaseous component of the Earth atmosphere and a chemical reagent participating in the combustion reactions, were addressed in the era of pneumatic chemistry.

The alcohol thermometer or spirit thermometer has a similar construction and theory of operation as a mercury-in-glass thermometer. However, the thermometric fluid of an alcohol thermometer is less toxic and evaporates quickly making it a safer alternative to mercury thermometers. The ethanol version is the most widely used due to the low cost and relatively low hazard posed by the liquid in case of breakage.