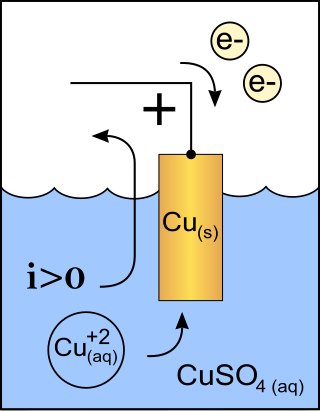
A cathode is the electrode from which a conventional current leaves a polarized electrical device such as a lead-acid battery. This definition can be recalled by using the mnemonic CCD for Cathode Current Departs. A conventional current describes the direction in which positive charges move. Electrons have a negative electrical charge, so the movement of electrons is opposite to that of the conventional current flow. Consequently, the mnemonic cathode current departs also means that electrons flow into the device's cathode from the external circuit. For example, the end of a household battery marked with a + (plus) is the cathode.

An arc lamp or arc light is a lamp that produces light by an electric arc.
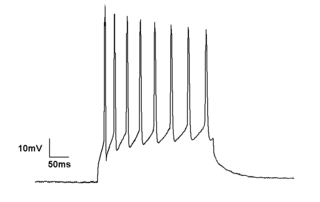
Electrophysiology is the branch of physiology that studies the electrical properties of biological cells and tissues. It involves measurements of voltage changes or electric current or manipulations on a wide variety of scales from single ion channel proteins to whole organs like the heart. In neuroscience, it includes measurements of the electrical activity of neurons, and, in particular, action potential activity. Recordings of large-scale electric signals from the nervous system, such as electroencephalography, may also be referred to as electrophysiological recordings. They are useful for electrodiagnosis and monitoring.

The voltage clamp is an experimental method used by electrophysiologists to measure the ion currents through the membranes of excitable cells, such as neurons, while holding the membrane voltage at a set level. A basic voltage clamp will iteratively measure the membrane potential, and then change the membrane potential (voltage) to a desired value by adding the necessary current. This "clamps" the cell membrane at a desired constant voltage, allowing the voltage clamp to record what currents are delivered. Because the currents applied to the cell must be equal to the current going across the cell membrane at the set voltage, the recorded currents indicate how the cell reacts to changes in membrane potential. Cell membranes of excitable cells contain many different kinds of ion channels, some of which are voltage-gated. The voltage clamp allows the membrane voltage to be manipulated independently of the ionic currents, allowing the current–voltage relationships of membrane channels to be studied.

The patch clamp technique is a laboratory technique in electrophysiology used to study ionic currents in individual isolated living cells, tissue sections, or patches of cell membrane. The technique is especially useful in the study of excitable cells such as neurons, cardiomyocytes, muscle fibers, and pancreatic beta cells, and can also be applied to the study of bacterial ion channels in specially prepared giant spheroplasts.

High-intensity discharge lamps are a type of electrical gas-discharge lamp which produces light by means of an electric arc between tungsten electrodes housed inside a translucent or transparent fused quartz or fused alumina arc tube. This tube is filled with noble gas and often also contains suitable metal or metal salts. The noble gas enables the arc's initial strike. Once the arc is started, it heats and evaporates the metallic admixture. Its presence in the arc plasma greatly increases the intensity of visible light produced by the arc for a given power input, as the metals have many emission spectral lines in the visible part of the spectrum. High-intensity discharge lamps are a type of arc lamp.
In electromagnetism, current sources and sinks are analysis formalisms which distinguish points, areas, or volumes through which electric current enters or exits a system. While current sources or sinks are abstract elements used for analysis, generally they have physical counterparts in real-world applications; e.g. the anode or cathode in a battery. In all cases, each of the opposing terms may refer to the same object, depending on the perspective of the observer and the sign convention being used; there is no intrinsic difference between a source and a sink.
The sucrose gap technique is used to create a conduction block in nerve or muscle fibers. A high concentration of sucrose is applied to the extracellular space, which prevents the correct opening and closing of sodium and potassium channels, increasing resistance between two groups of cells. It was originally developed by Robert Stämpfli for recording action potentials in nerve fibers, and is particularly useful for measuring irreversible or highly variable pharmacological modifications of channel properties since untreated regions of membrane can be pulled into the node between the sucrose regions.

Voltammetry is a category of electroanalytical methods used in analytical chemistry and various industrial processes. In voltammetry, information about an analyte is obtained by measuring the current as the potential is varied. The analytical data for a voltammetric experiment comes in the form of a voltammogram, which plots the current produced by the analyte versus the potential of the working electrode.
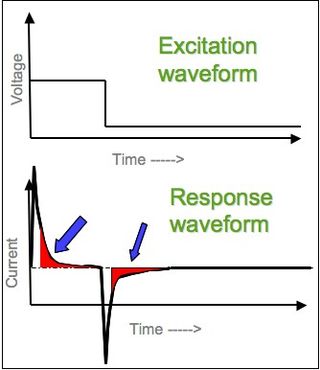
In electrochemistry, chronoamperometry is an analytical technique in which the electric potential of the working electrode is stepped and the resulting current from faradaic processes occurring at the electrode is monitored as a function of time. The functional relationship between current response and time is measured after applying single or double potential step to the working electrode of the electrochemical system. Limited information about the identity of the electrolyzed species can be obtained from the ratio of the peak oxidation current versus the peak reduction current. However, as with all pulsed techniques, chronoamperometry generates high charging currents, which decay exponentially with time as any RC circuit. The Faradaic current - which is due to electron transfer events and is most often the current component of interest - decays as described in the Cottrell equation. In most electrochemical cells, this decay is much slower than the charging decay-cells with no supporting electrolyte are notable exceptions. Most commonly a three-electrode system is used. Since the current is integrated over relatively longer time intervals, chronoamperometry gives a better signal-to-noise ratio in comparison to other amperometric techniques.
Neural engineering is a discipline within biomedical engineering that uses engineering techniques to understand, repair, replace, or enhance neural systems. Neural engineers are uniquely qualified to solve design problems at the interface of living neural tissue and non-living constructs.
In neuroscience, single-unit recordings provide a method of measuring the electro-physiological responses of a single neuron using a microelectrode system. When a neuron generates an action potential, the signal propagates down the neuron as a current which flows in and out of the cell through excitable membrane regions in the soma and axon. A microelectrode is inserted into the brain, where it can record the rate of change in voltage with respect to time. These microelectrodes must be fine-tipped, impedance matching; they are primarily glass micro-pipettes, metal microelectrodes made of platinum, tungsten, iridium or even iridium oxide. Microelectrodes can be carefully placed close to the cell membrane, allowing the ability to record extracellularly.
Local field potentials (LFP) are transient electrical signals generated in nerves and other tissues by the summed and synchronous electrical activity of the individual cells in that tissue. LFP are "extracellular" signals, meaning that they are generated by transient imbalances in ion concentrations in the spaces outside the cells, that result from cellular electrical activity. LFP are 'local' because they are recorded by an electrode placed nearby the generating cells. As a result of the Inverse-square law, such electrodes can only 'see' potentials in a spatially limited radius. They are 'potentials' because they are generated by the voltage that results from charge separation in the extracellular space. They are 'field' because those extracellular charge separations essentially create a local electric field. LFP are typically recorded with a high-impedance microelectrode placed in the midst of the population of cells generating it. They can be recorded, for example, via a microelectrode placed in the brain of a human or animal subject, or in an in vitro brain thin slice.
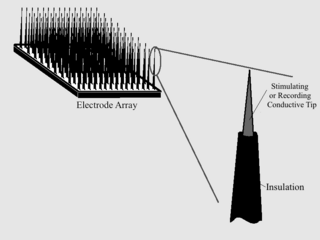
A microelectrode is an electrode used in electrophysiology either for recording neural signals or for the electrical stimulation of nervous tissue. Pulled glass pipettes with tip diameters of 0.5 μm or less are usually filled with 3 molars potassium chloride solution as the electrical conductor. When the tip penetrates a cell membrane the lipids in the membrane seal onto the glass, providing an excellent electrical connection between the tip and the interior of the cell, which is apparent because the microelectrode becomes electrically negative compared to the extracellular solution. There are also microelectrodes made with insulated metal wires, made from inert metals with high Young modulus such as tungsten, stainless steel, or platinum-iridium alloy and coated with glass or polymer insulator with exposed conductive tips. These are mostly used for recording from the external side of the cell membrane. More recent advances in lithography have produced silicon-based microelectrodes.
An ultramicroelectrode (UME) is a working electrode used in a voltammetry. The small size of UME give them large diffusion layers and small overall currents. These features allow UME to achieve useful steady-state conditions and very high scan rates (V/s) with limited distortion. UME were developed independently by Wightman and Fleischmann around 1980. Small current at UME enables electrochemical measurements in low conductive media, where voltage drop associated with high solution resistance makes these experiments difficult for conventional electrodes. Furthermore, small voltage drop at UME leads to a very small voltage distortion at the electrode-solution interface which allows using two-electrode setup in voltammetric experiment instead of conventional three-electrode setup.
Microelectrode arrays (MEAs) are devices that contain multiple microelectrodes through which neural signals are obtained or delivered, essentially serving as neural interfaces that connect neurons to electronic circuitry. There are two general classes of MEAs: implantable MEAs, used in vivo, and non-implantable MEAs, used in vitro.
Neurostimulation is the purposeful modulation of the nervous system's activity using invasive or non-invasive means. Neurostimulation usually refers to the electromagnetic approaches to neuromodulation.
A liquid metal electrode is an electrode that uses a liquid metal, such as mercury, Galinstan, and NaK. They can be used in electrocapillarity, voltammetry, and impedance measurements.
As with any material implanted in the body, it is important to minimize or eliminate foreign body response and maximize effectual integration. Neural implants have the potential to increase the quality of life for patients with such disabilities as Alzheimer's, Parkinson's, epilepsy, depression, and migraines. With the complexity of interfaces between a neural implant and brain tissue, adverse reactions such as fibrous tissue encapsulation that hinder the functionality, occur. Surface modifications to these implants can help improve the tissue-implant interface, increasing the lifetime and effectiveness of the implant.
A chronic electrode implant is an electronic device implanted chronically into the brain or other electrically excitable tissue. It may record electrical impulses in the brain or may stimulate neurons with electrical impulses from an external source.








