Related Research Articles
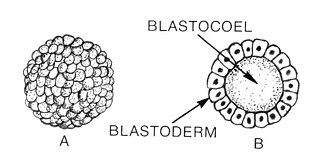
Blastulation is the stage in early animal embryonic development that produces the blastula. The blastula is a hollow sphere of cells known as blastomeres surrounding an inner fluid-filled cavity called the blastocoel. Embryonic development begins with a sperm fertilizing an egg cell to become a zygote, which undergoes many cleavages to develop into a ball of cells called a morula. Only when the blastocoel is formed does the early embryo become a blastula. The blastula precedes the formation of the gastrula in which the germ layers of the embryo form.

A germ cell is any biological cell that gives rise to the gametes of an organism that reproduces sexually. In many animals, the germ cells originate in the primitive streak and migrate via the gut of an embryo to the developing gonads. There, they undergo meiosis, followed by cellular differentiation into mature gametes, either eggs or sperm. Unlike animals, plants do not have germ cells designated in early development. Instead, germ cells can arise from somatic cells in the adult, such as the floral meristem of flowering plants.
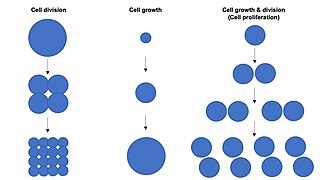
Cell growth refers to an increase in the total mass of a cell, including both cytoplasmic, nuclear and organelle volume. Cell growth occurs when the overall rate of cellular biosynthesis is greater than the overall rate of cellular degradation.

Drosophila embryogenesis, the process by which Drosophila embryos form, is a favorite model system for genetics and developmental biology. The study of its embryogenesis unlocked the century-long puzzle of how development was controlled, creating the field of evolutionary developmental biology. The small size, short generation time, and large brood size make it ideal for genetic studies. Transparent embryos facilitate developmental studies. Drosophila melanogaster was introduced into the field of genetic experiments by Thomas Hunt Morgan in 1909.
SOX genes encode a family of transcription factors that bind to the minor groove in DNA, and belong to a super-family of genes characterized by a homologous sequence called the HMG-box. This HMG box is a DNA binding domain that is highly conserved throughout eukaryotic species. Homologues have been identified in insects, nematodes, amphibians, reptiles, birds and a range of mammals. However, HMG boxes can be very diverse in nature, with only a few amino acids being conserved between species.
The cells that give rise to the gametes are often set aside during embryonic cleavage. During development, these cells will differentiate into primordial germ cells, migrate to the location of the gonad, and form the germline of the animal.
Piwi genes were identified as regulatory proteins responsible for stem cell and germ cell differentiation. Piwi is an abbreviation of P-elementInduced WImpy testis in Drosophila. Piwi proteins are highly conserved RNA-binding proteins and are present in both plants and animals. Piwi proteins belong to the Argonaute/Piwi family and have been classified as nuclear proteins. Studies on Drosophila have also indicated that Piwi proteins have slicer activity conferred by the presence of the Piwi domain. In addition, Piwi associates with heterochromatin protein 1, an epigenetic modifier, and piRNA-complementary sequences. These are indications of the role Piwi plays in epigenetic regulation. Piwi proteins are also thought to control the biogenesis of piRNA as many Piwi-like proteins contain slicer activity which would allow Piwi proteins to process precursor piRNA into mature piRNA.
oskar is a gene required for the development of the Drosophila embryo. It defines the posterior pole during early embryogenesis. Its two isoforms, short and long, play different roles in Drosophila embryonic development. oskar was named after the main character from the Günter Grass novel The Tin Drum, who refuses to grow up.
Faint little ball (flb) is a Drosophila gene that encodes the Drosophila epidermal growth factor receptor (DER) homolog. The gene is also called torpedo and Ellipse. The gene is located at 3-26 of the Drosophila melanogaster genome. It is named faint little ball because when the gene is mutated the embryo forms a ball of dorsal hypoderm. flb is necessary for several processes to occur during embryonic development, specifically in central nervous system development. It is expressed as quickly as 4 hours after fertilization of the egg. The peak of expression of the flb gene is between 4–8 hours into development. In all processes that are facilitated by flb the same signal transduction pathway is used. Drosophila EGF receptor is involved in the development of embryos as well as larvae/pupae's wings, eyes, legs and ovaries.
Maternal to zygotic transition is the stage in embryonic development during which development comes under the exclusive control of the zygotic genome rather than the maternal (egg) genome. The egg contains stored maternal genetic material mRNA which controls embryo development until the onset of MZT. After MZT the diploid embryo takes over genetic control. This requires both zygotic genome activation (ZGA) and degradation of maternal products. This process is important because it is the first time that the new embryonic genome is utilized and the paternal and maternal genomes are used in combination. The zygotic genome now drives embryo development.
Vasa is an RNA binding protein with an ATP-dependent RNA helicase that is a member of the DEAD box family of proteins. The vasa gene, is essential for germ cell development and was first identified in Drosophila melanogaster, but has since been found to be conserved in a variety of vertebrates and invertebrates including humans. The Vasa protein is found primarily in germ cells in embryos and adults, where it is involved in germ cell determination and function, as well as in multipotent stem cells, where its exact function is unknown.

Female-specific protein transformer is a protein that in Drosophila melanogaster is encoded by the tra gene. Unlike the related tra2 protein, it is only produced in females.

Rhomboid-related protein 2 is a protein that in humans is encoded by the RHBDL2 gene.

Homeotic protein bicoid is encoded by the bcd maternal effect gene in Drosophilia. Homeotic protein bicoid concentration gradient patterns the anterior-posterior (A-P) axis during Drosophila embryogenesis. Bicoid was the first protein demonstrated to act as a morphogen. Although bicoid is important for the development of Drosophila and other higher dipterans, it is absent from most other insects, where its role is accomplished by other genes.
Vrille (vri) is a bZIP transcription factor found on chromosome 2 in Drosophila melanogaster. Vrille mRNA and protein product (VRI) oscillate predictably on a 24-hour timescale and interact with other circadian clock genes to regulate circadian rhythms in Drosophila. It is also a regulator in embryogenesis; it is expressed in multiple cell types during multiple stages in development, coordinating embryonic dorsal/ventral polarity, wing-vein differentiation, and ensuring tracheal integrity. It is also active in the embryonic gut but the precise function there is unknown. Mutations in vri alter circadian period and cause circadian arrhythmicity and developmental defects in Drosophila.

Spätzle or spaetzle is an evolutionarily-conserved arthropod protein first identified in Drosophila melanogaster. It plays a role in embryonic development and in the insect innate immune response. The name was coined by the Nobel laureate Christiane Nüsslein-Volhard after the Spätzle noodle-like form of homozygous mutant fly larvae.
Anne Ephrussi is a French developmental and molecular biologist. Her research is focussed on the study of post-transcriptional regulations such as mRNA localization and translation control in molecular biology as well as the establishment of polarity axes in cell and developmental biology Ephrussi Group - RNA localisation and localised translation in development - EMBL. She is Head of the Developmental Biology Unit and director of the EMBL International Centre for Advanced Training (EICAT) program at the European Molecular Biology Laboratory (EMBL).
Susan Strome is a Distinguished Professor of Molecular, Cell, and Developmental Biology at the University of California Santa Cruz. Strome received a B.A. degree in Chemistry from University of New Mexico and a Ph.D. in Biochemistry from the University of Washington, as well as post-graduate work at the University of Colorado Boulder. Strome is a member of the American Academy of Arts and Sciences and the National Academy of Sciences.

Primordial germ cell (PGC) migration is the process of distribution of primordial germ cells throughout the embryo during embryogenesis.
Elizabeth Gavis is an American biologist who is the Damon B. Pfeiffer Professor of Life Sciences, at Princeton University. Gavis served as the President of the North American Drosophila Board of Directors in 2011.
References
- ↑ Willmer, P. G. (1990). Invertebrate Relationships : Patterns in Animal Evolution. Cambridge University Press, Cambridge.
- ↑ Saito, Kuniaki (2013). "The epigenetic regulation of transposable elements by PIWI-interacting RNAs in Drosophila". Genes & Genetic Systems. 88 (1): 9–17. doi: 10.1266/ggs.88.9 . ISSN 1341-7568.
- ↑ Kobayashi, Satoru; Okada, Masukichi (August 1989). "Mitochondrial lrRNA sequences restore pole cell-forming ability to UV-sterilized embryos". Cell Differentiation and Development. 27: 123. doi:10.1016/0922-3371(89)90382-1. ISSN 0922-3371.
- ↑ Harria, Adam; Macdonald, Paul (2001). "aubergine encodes a Drosophila polar granule component required for pole cell formation and related to eIF2C". Development. 128: 2823–2832.
- ↑ Jakobsen, Rasmus Kragh; Ono, Shin; Powers, James C.; DeLotto, Robert (2004-12-18). "Fluorescently labeled inhibitors detect localized serine protease activities in Drosophila melanogaster pole cells, embryos, and ovarian egg chambers". Histochemistry and Cell Biology. 123 (1): 51–60. doi:10.1007/s00418-004-0734-5. ISSN 0948-6143. PMID 15609041.
- ↑ Lin, Haifan; Wolfner, Mariana F. (January 1991). "The Drosophila maternal-effect gene fs(1)Ya encodes a cell cycle-dependent nuclear envelope component required for embryonic mitosis". Cell. 64 (1): 49–62. doi:10.1016/0092-8674(91)90208-g. ISSN 0092-8674.
- ↑ Callaini, Giuliano; Riparbelli, Maria Giovanna; Dallai, Romano (August 1995). "Pole Cell Migration through the Gut Wall of the Drosophila Embryo: Analysis of Cell Interactions". Developmental Biology. 170 (2): 365–375. doi: 10.1006/dbio.1995.1222 . ISSN 0012-1606. PMID 7649369.