Related Research Articles

Progestogens, also sometimes written progestagens or gestagens, are a class of natural or synthetic steroid hormones that bind to and activate the progesterone receptors (PR). Progesterone is the major and most important progestogen in the body. The progestogens are named for their function in maintaining pregnancy, although they are also present at other phases of the estrous and menstrual cycles.
Neurosteroids, also known as neuroactive steroids, are endogenous or exogenous steroids that rapidly alter neuronal excitability through interaction with ligand-gated ion channels and other cell surface receptors. The term neurosteroid was coined by the French physiologist Étienne-Émile Baulieu and refers to steroids synthesized in the brain. The term, neuroactive steroid refers to steroids that can be synthesized in the brain, or are synthesized by an endocrine gland, that then reach the brain through the bloodstream and have effects on brain function. The term neuroactive steroids was first coined in 1992 by Steven Paul and Robert Purdy. In addition to their actions on neuronal membrane receptors, some of these steroids may also exert effects on gene expression via nuclear steroid hormone receptors. Neurosteroids have a wide range of potential clinical applications from sedation to treatment of epilepsy and traumatic brain injury. Ganaxolone, a synthetic analog of the endogenous neurosteroid allopregnanolone, is under investigation for the treatment of epilepsy.

Etiocholanolone, also known as 5β-androsterone, as well as 3α-hydroxy-5β-androstan-17-one or etiocholan-3α-ol-17-one, is an etiocholane (5β-androstane) steroid as well as an endogenous 17-ketosteroid that is produced from the metabolism of testosterone. It causes fever, immunostimulation, and leukocytosis, and is used to evaluate adrenal cortex function, bone marrow performance, and in neoplastic disease to stimulate the immune system. Etiocholanolone is also known to be an inhibitory androstane neurosteroid, acting as a positive allosteric modulator of the GABAA receptor, and possesses anticonvulsant effects. The unnatural enantiomer of etiocholanolone is more potent as a positive allosteric modulator of GABAA receptors and as an anticonvulsant than the natural form.

Allopregnanolone is a naturally occurring neurosteroid which is made in the body from the hormone progesterone. As a medication, allopregnanolone is referred to as brexanolone, sold under the brand name Zulresso, and used to treat postpartum depression. It is given by injection into a vein.

Alfaxalone, also known as alphaxalone or alphaxolone and sold under the brand name Alfaxan, is a neuroactive steroid and general anesthetic which is used currently in veterinary practice as an induction agent for anesthesia and as an injectable anesthetic. Though it is more expensive than other induction agents, it often preferred due to the lack of depressive effects on the cardiovascular system. The most common side effect seen in current veterinary practice is respiratory depression when Alfaxan is administered concurrently with other sedative and anesthetic drugs; when premedications aren't given, veterinary patients also become agitated and hypersensitive when waking up.
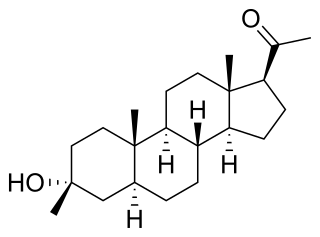
Ganaxolone, sold under the brand name Ztalmy, is a medication used to treat seizures in people with cyclin-dependent kinase-like 5 (CDKL5) deficiency disorder (CDD).
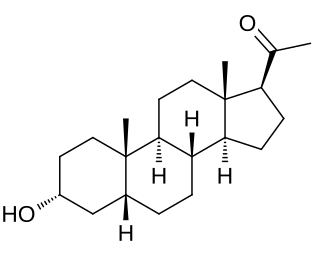
Pregnanolone, also known as eltanolone, is an endogenous inhibitory neurosteroid which is produced in the body from progesterone. It is closely related to allopregnanolone, which has similar properties.
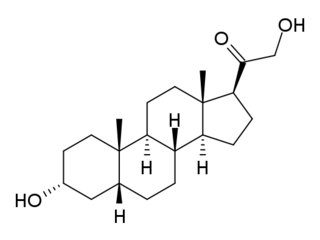
Tetrahydrodeoxycorticosterone, also referred to as allotetrahydrocorticosterone, is an endogenous neurosteroid. It is synthesized from the adrenal hormone deoxycorticosterone by the action of two enzymes, 5α-reductase type I and 3α-hydroxysteroid dehydrogenase. THDOC is a potent positive allosteric modulator of the GABAA receptor, and has sedative, anxiolytic and anticonvulsant effects. Changes in the normal levels of this steroid particularly during pregnancy and menstruation may be involved in some types of epilepsy and premenstrual syndrome, as well as stress, anxiety and depression.

5α-Dihydroprogesterone is an endogenous progestogen and neurosteroid that is synthesized from progesterone. It is also an intermediate in the synthesis of allopregnanolone and isopregnanolone from progesterone.

In pharmacology, GABAA receptor positive allosteric modulators, also known as GABAkines or GABAA receptor potentiators, are positive allosteric modulator (PAM) molecules that increase the activity of the GABAA receptor protein in the vertebrate central nervous system.
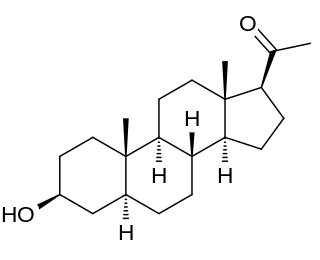
Isopregnanolone, also known as isoallopregnanolone and epiallopregnanolone, as well as sepranolone (INN), and as 3β-hydroxy-5α-pregnan-20-one or 3β,5α-tetrahydroprogesterone (3β,5α-THP), is an endogenous neurosteroid and a natural 3β-epimer of allopregnanolone. It has been reported to act as a subunit-selective negative allosteric modulator of the GABAA receptor, and antagonizes in animals and humans some but not all of the GABAA receptor-mediated effects of allopregnanolone, such as anesthesia, sedation, and reduced saccadic eye movements, but not learning impairment. Isopregnanolone has no hormonal effects and appears to have no effect on the GABAA receptor by itself; it selectively antagonizes allopregnanolone and does not affect the effects of other types of GABAA receptor positive allosteric modulators such as benzodiazepines or barbiturates.
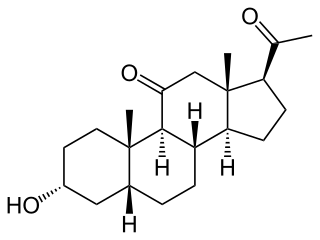
Renanolone (INN), or 11-ketopregnanolone, also known as 5β-pregnan-3α-ol-11,20-dione, is a synthetic neuroactive steroid which is described as a general anesthetic but was never introduced for clinical use. Its isomers, alfaxolone and alfadolone, are also general anesthetics, and are known to act as positive allosteric modulators of the GABAA receptor, a property which is likely the case for renanolone as well.

Epipregnanolone, also known as 3β-hydroxy-5β-pregnan-20-one, 3β,5β-tetrahydroprogesterone, or 3β,5β-THP, is an endogenous neurosteroid. It acts as a negative allosteric modulator of the GABAA receptor and reverses the effects of potentiators like allopregnanolone. Epipregnanolone is biosynthesized from progesterone by the actions of 5β-reductase and 3β-hydroxysteroid dehydrogenase, with 5β-dihydroprogesterone as the intermediate in this two-step transformation.

5β-Dihydroprogesterone is an endogenous neurosteroid and an intermediate in the biosynthesis of pregnanolone and epipregnanolone from progesterone. It is synthesized from progesterone by the enzyme 5β-reductase.
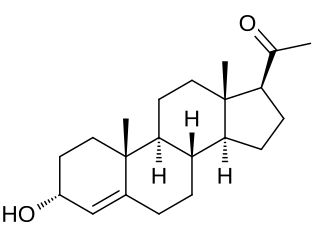
3α-Dihydroprogesterone (3α-DHP), also known as 3α-hydroxyprogesterone, as well as pregn-4-en-3α-ol-20-one, is an endogenous neurosteroid. It is biosynthesized by 3α-hydroxysteroid dehydrogenase from progesterone. 3α-DHP has been found to act as a positive allosteric modulator of the GABAA receptor and is described as being as active as allopregnanolone in regard to this action. In accordance, it has anxiolytic effects in animals. 3α-DHP has also been found to inhibit the secretion of follicle-stimulating hormone (FSH) from the rat pituitary gland, demonstrating possible antigonadotropic properties. Unlike the case of most other inhibitory neurosteroids, 3α-DHP production is not blocked by 5α-reductase inhibitors like finasteride. No data were available on the progestogenic activity of 3α-DHP as of 1977. Levels of 5α-DHP have been quantified.

3β-Dihydroprogesterone (3β-DHP), also known as 3β-hydroxyprogesterone, or pregn-4-en-3β-ol-20-one, is an endogenous steroid. It is biosynthesized by 3β-hydroxysteroid dehydrogenase from progesterone. Unlike 3α-dihydroprogesterone (3α-DHP), 3β-DHP does not act as a positive allosteric modulator of the GABAA receptor, which is in accordance with the fact that other 3β-hydroxylated progesterone metabolites such as isopregnanolone and epipregnanolone similarly do not act as potentiators of this receptor and instead inhibit it as well as reverse the effects of potentiators like allopregnanolone. 3β-DHP has been reported to possess about the same potency as progesterone in a bioassay of progestogenic activity, whereas 3α-DHP was not assessed.

Allopregnanediol, or 5α-pregnane-3α,20α-diol, is an endogenous metabolite of progesterone and allopregnanolone and an isomer of pregnanediol (5β-pregnan-3α,20α-diol). It has been found to act like a partial agonist of an allosteric site of the GABA receptor and hence might play a biological role as a neurosteroid. It has also been found to act as an agonist of the human pregnane X receptor, albeit with an EC50 that is more than an order of magnitude lower than that of other endogenous pregnanes like pregnenolone, pregnanediol, allopregnanedione, and allopregnanolone.

Zuranolone is an investigational medication which is under development by SAGE Therapeutics for the treatment of depressive disorders and a variety of other indications. It is a synthetic, orally active, inhibitory pregnane neurosteroid, and acts as a positive allosteric modulator of the GABAA receptor. The drug was developed as an improvement of brexanolone (allopregnanolone) with high oral bioavailability and a biological half-life suitable for once-daily administration. Its half-life is around 16 to 23 hours, compared to approximately 9 hours for brexanolone. As of December 2022, zuranolone is in preregistration for major depressive disorder and postpartum depression, phase III clinical trials for insomnia, and phase II clinical studies for bipolar depression, essential tremor, and Parkinson's disease. Zuranolone was also under investigation for treatment of dyskinesias and seizures, but no further development has been reported for these indications.

5α-Pregnane-3α,17α-diol-20-one, also known as 17α-hydroxyallopregnanolone (17-OH-allo) is an endogenous steroid.
References
- ↑ Havlíková, Helena; Hill, Martin; Kancheva, Lyudmila; Vrbíková, Jana; Pouzar, Vladimír; Černý, Ivan; Kancheva, Radmila; Stárka, Luboslav (2006). "Serum Profiles of Free and Conjugated Neuroactive Pregnanolone Isomers in Nonpregnant Women of Fertile Age". The Journal of Clinical Endocrinology & Metabolism. 91 (8): 3092–3099. doi: 10.1210/jc.2005-2785 . ISSN 0021-972X. PMID 16720657.
- ↑ Shannon, E. E. (2005). "Characterization of the Discriminative Stimulus Effects of the Neuroactive Steroid Pregnanolone in DBA/2J and C57BL/6J Inbred Mice". Journal of Pharmacology and Experimental Therapeutics. 314 (2): 675–685. doi:10.1124/jpet.104.082644. ISSN 0022-3565. PMID 15857945. S2CID 717856.