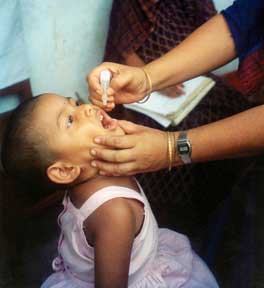
Polio vaccines are vaccines used to prevent poliomyelitis (polio). Two types are used: an inactivated poliovirus given by injection (IPV) and a weakened poliovirus given by mouth (OPV). The World Health Organization (WHO) recommends all children be fully vaccinated against polio. The two vaccines have eliminated polio from most of the world, and reduced the number of cases reported each year from an estimated 350,000 in 1988 to 33 in 2018.
The National Institute of Allergy and Infectious Diseases is one of the 27 institutes and centers that make up the National Institutes of Health (NIH), an agency of the United States Department of Health and Human Services (HHS). NIAID's mission is to conduct basic and applied research to better understand, treat, and prevent infectious, immunologic, and allergic diseases.
The Vaccine Safety Datalink Project (VSD) was established in 1990 by the United States Centers for Disease Control and Prevention (CDC) to study the adverse effects of vaccines.

The Ministry of Health of Chile, also known as MINSAL, is the cabinet-level administrative office in charge of planning, directing, coordinating, executing, controlling and informing the public health policies formulated by the President of Chile. Notably, all employees pay 7% of their monthly income to FONASA, the funding branch of the Chilean Ministry of Health.

Sinovac Biotech Ltd. is a Chinese biopharmaceutical company based in Haidian District, Beijing that focuses on the research, development, manufacture, and commercialization of vaccines that protect against human infectious diseases. The company was listed on the Nasdaq but the exchange halted Sinovac's trading in February 2019 due to a proxy fight. The company has faced bribery probes in China. Its COVID-19 vaccine was also the target of a covert disinformation campaign by the US government.

Tick-borne encephalitis vaccine is a vaccine used to prevent tick-borne encephalitis (TBE). The disease is most common in Central and Eastern Europe, and Northern Asia. More than 87% of people who receive the vaccine develop immunity. It is not useful following the bite of an infected tick. It is given by injection into a muscle.

Measles vaccine protects against becoming infected with measles. Nearly all of those who do not develop immunity after a single dose develop it after a second dose. When the rate of vaccination within a population is greater than 92%, outbreaks of measles typically no longer occur; however, they may occur again if the rate of vaccination decreases. The vaccine's effectiveness lasts many years. It is unclear if it becomes less effective over time. The vaccine may also protect against measles if given within a couple of days after exposure to measles.

Yellow fever vaccine is a vaccine that protects against yellow fever. Yellow fever is a viral infection that occurs in Africa and South America. Most people begin to develop immunity within ten days of vaccination and 99% are protected within one month, and this appears to be lifelong. The vaccine can be used to control outbreaks of disease. It is given either by injection into a muscle or just under the skin.

Katherine "Kate" L. O'Brien is a Canadian American pediatric infectious disease physician, epidemiologist, and vaccinologist who specializes in the areas of pneumococcal epidemiology, pneumococcal vaccine trials and impact studies, and surveillance for pneumococcal disease. She is also known as an expert in infectious diseases in American Indian populations. O’Brien is currently the Director of the World Health Organization's Department of Immunization, Vaccines and Biologicals.
The International Vaccine Institute (IVI) is a non-profit, autonomous international organization established with the mandate of making vaccines available to all. Collaborating closely with the global scientific community, public health entities, governments, and industry stakeholders, IVI focuses on vaccine research and deployment. This includes conducting new vaccine designs in laboratories, advancing vaccine development and assessment in real-world settings, and facilitating the sustainable integration of vaccines in regions where they are most urgently required.

The Academy of Military Medical Sciences (AMMS) of the People's Liberation Army Academy of Military Sciences is a Chinese military medical research institute. It was established in Shanghai in 1951. It has been based in Beijing since 1958.

Chen Wei is a Chinese epidemiologist and virologist specializing in biodefense, and is currently working as a researcher and doctoral advisor at the Academy of Military Medical Sciences, and is an Academician of the Chinese Academy of Engineering (CAE). In September 2021, The Globe and Mail reported that Chen is also a major general in China's People's Liberation Army.

The 1957–1958 Asian flu pandemic was a global pandemic of influenza A virus subtype H2N2 that originated in Guizhou in Southern China. The number of excess deaths caused by the pandemic is estimated to be 1–4 million around the world, making it one of the deadliest pandemics in history. A decade later, a reassorted viral strain H3N2 further caused the Hong Kong flu pandemic (1968–1969).
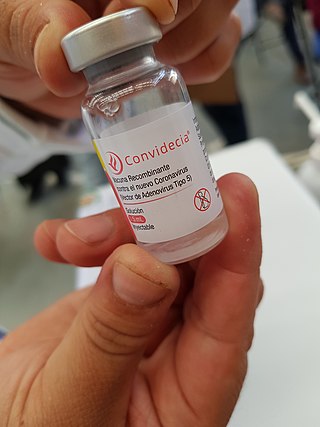
AD5-nCOV, trade-named Convidecia, is a single-dose viral vector vaccine for COVID-19 that is also used as an inhaled booster. It was developed by CanSino Biologics, with Phase III trials conducted in Argentina, Chile, Mexico, Pakistan, Russia, and Saudi Arabia with 40,000 participants.

CanSino Biologics, often abbreviated as CanSinoBIO, is a Chinese vaccine company.

Vaccine diplomacy, a form of medical diplomacy, is the use of vaccines to improve a country's diplomatic relationship and influence of other countries. Meanwhile, vaccine diplomacy also "means a set of diplomatic measures taken to ensure access to the best practices in the development of potential vaccines, to enhance bilateral and/or multilateral cooperation between countries in conducting joint R&D, and, in the case of the announcement of production, to ensure the signing of a contract for the purchase of the vaccine at the shortest term." Although primary discussed in the context of the supply of COVID-19 vaccines, it also played a part in the distribution of the smallpox vaccine.

CoronaVac, also known as the Sinovac COVID-19 vaccine, was a whole inactivated virus COVID-19 vaccine developed by the Chinese company Sinovac Biotech. It was phase III clinically trialled in Brazil, Chile, Indonesia, the Philippines, and Turkey and relies on traditional technology similar to other inactivated-virus COVID-19 vaccines, such as the Sinopharm BIBP vaccine, another Chinese vaccine, and Covaxin, an Indian vaccine. CoronaVac does not need to be frozen, and both the final product and the raw material for formulating CoronaVac can be transported refrigerated at 2–8 °C (36–46 °F), the temperatures at which flu vaccines are kept.
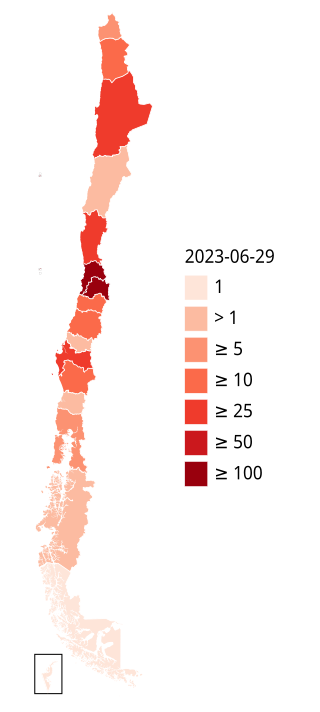
The 2022–2023 mpox outbreak in Chile is a part of the outbreak of human mpox caused by the West African clade of the monkeypox virus. The outbreak reached Chile on 17 June 2022.
















