Health Canada is the department of the Government of Canada responsible for national health policy. The department itself is also responsible for numerous federal health-related agencies, including the Canadian Food Inspection Agency (CFIA) and the Public Health Agency of Canada (PHAC), among others. These organizations help to ensure compliance with federal law in a variety of healthcare, agricultural, and pharmaceutical activities. This responsibility also involves extensive collaboration with various other federal- and provincial-level organizations in order to ensure the safety of food, health, and pharmaceutical products—including the regulation of health research and pharmaceutical manufacturing/testing facilities.
The Dietary Reference Intake (DRI) is a system of nutrition recommendations from the National Academy of Medicine (NAM) of the National Academies. It was introduced in 1997 in order to broaden the existing guidelines known as Recommended Dietary Allowances. The DRI values differ from those used in nutrition labeling on food and dietary supplement products in the U.S. and Canada, which uses Reference Daily Intakes (RDIs) and Daily Values (%DV) which were based on outdated RDAs from 1968 but were updated as of 2016.
Pharmacovigilance, also known as drug safety, is the pharmaceutical science relating to the "collection, detection, assessment, monitoring, and prevention" of adverse effects with pharmaceutical products. The etymological roots for the word "pharmacovigilance" are: pharmakon and vigilare. As such, pharmacovigilance heavily focuses on adverse drug reactions (ADR), which are defined as any response to a drug which is noxious and unintended, including lack of efficacy. Medication errors such as overdose, and misuse and abuse of a drug as well as drug exposure during pregnancy and breastfeeding, are also of interest, even without an adverse event, because they may result in an adverse drug reaction.
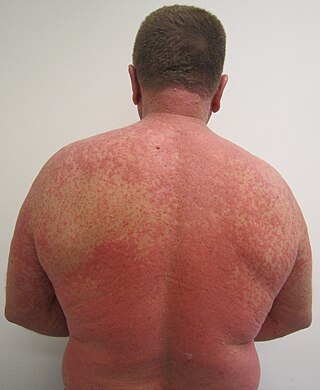
An adverse drug reaction (ADR) is a harmful, unintended result caused by taking medication. ADRs may occur following a single dose or prolonged administration of a drug or may result from the combination of two or more drugs. The meaning of this term differs from the term "side effect" because side effects can be beneficial as well as detrimental. The study of ADRs is the concern of the field known as pharmacovigilance. An adverse event (AE) refers to any unexpected and inappropriate occurrence at the time a drug is used, whether or not the event is associated with the administration of the drug. An ADR is a special type of AE in which a causative relationship can be shown. ADRs are only one type of medication-related harm. Another type of medication-related harm type includes not taking prescribed medications, known as non-adherence. Non-adherence to medications can lead to death and other negative outcomes. Adverse drug reactions require the use of a medication.
An adverse effect is an undesired harmful effect resulting from a medication or other intervention, such as surgery. An adverse effect may be termed a "side effect", when judged to be secondary to a main or therapeutic effect. The term complication is similar to adverse effect, but the latter is typically used in pharmacological contexts, or when the negative effect is expected or common. If the negative effect results from an unsuitable or incorrect dosage or procedure, this is called a medical error and not an adverse effect. Adverse effects are sometimes referred to as "iatrogenic" because they are generated by a physician/treatment. Some adverse effects occur only when starting, increasing or discontinuing a treatment. Using a drug or other medical intervention which is contraindicated may increase the risk of adverse effects. Adverse effects may cause complications of a disease or procedure and negatively affect its prognosis. They may also lead to non-compliance with a treatment regimen. Adverse effects of medical treatment resulted in 142,000 deaths in 2013 up from 94,000 deaths in 1990 globally.
A poison control center is a medical service that is able to provide immediate, free, and expert treatment advice and assistance over the telephone in case of exposure to poisonous or hazardous substances. Poison control centers answer questions about potential poisons in addition to providing treatment management advice about household products, medicines, pesticides, plants, bites and stings, food poisoning, and fumes. In the US, more than 72% of poison exposure cases are managed by phone, greatly reducing the need for costly emergency department and doctor visits.

The Norwegian Institute of Public Health (NIPH) is a Norwegian government agency and research institute, and is Norway's national public health institute. It is subordinate to the Ministry of Health and Care Services. NIPH acts as a national competence institution in public health in a broad sense for governmental authorities, the health service, the judiciary, prosecuting authorities, politicians, the media and the general public, international organisations and foreign governments. The institute has around 1400 employees.
Patient safety is a discipline that emphasizes safety in health care through the prevention, reduction, reporting and analysis of error and other types of unnecessary harm that often lead to adverse patient events. The magnitude of avoidable adverse events, often known as patient safety incidents, experienced by patients was not well known until the 1990s, when multiple countries reported significant numbers of patients harmed and killed by medical errors. Recognizing that healthcare errors impact 1 in every 10 patients around the world, the World Health Organization (WHO) calls patient safety an endemic concern. Indeed, patient safety has emerged as a distinct healthcare discipline supported by an immature yet developing scientific framework. There is a significant transdisciplinary body of theoretical and research literature that informs the science of patient safety with mobile health apps being a growing area of research.
A patient safety organization (PSO) is a group, institution, or association that improves medical care by reducing medical errors. Common functions of patient safety organizations are data collection, analysis, reporting, education, funding, and advocacy. A PSO differs from a Federally designed Patient Safety Organization (PSO), which provides health care providers in the U.S. privilege and confidentiality protections for efforts to improve patient safety and the quality of patient care delivery
The Yellow Card Scheme is the United Kingdom's system for collecting information on suspected adverse drug reactions (ADRs) to medicines. The scheme allows the safety of the medicines and vaccines that are on the market to be monitored.

Tsan Yuk Hospital is maternity hospital is located on 30 Hospital Road, Sai Ying Pun on Hong Kong Island, is a public hospital in Hong Kong, It was specialising in obstetrics and gynaecology. It also operates as a teaching and training hospital for the medical and nursing students of Li Ka Shing Faculty of Medicine of the University of Hong Kong.

The Health Sciences Authority (HSA) is a statutory board under the Ministry of Health of the Government of Singapore. It is a multi-disciplinary agency responsible for applying medical, pharmaceutical, and scientific expertise to protect and advance public health and safety.
Healthcare in England is mainly provided by the National Health Service (NHS), a public body that provides healthcare to all permanent residents in England, that is free at the point of use. The body is one of four forming the UK National Health Service, as health is a devolved matter; there are differences with the provisions for healthcare elsewhere in the United Kingdom, and in England it is overseen by NHS England. Though the public system dominates healthcare provision in England, private health care and a wide variety of alternative and complementary treatments are available for those willing and able to pay.
Healthcare in Finland consists of a highly decentralized three-level publicly funded healthcare system and a much smaller private sector. Although the Ministry of Social Affairs and Health has the highest decision-making authority, specific healthcare precincts are responsible for providing healthcare to their residents as of 2023.
The following outline is provided as an overview of and topical guide to clinical research:

Healthcare in Sierra Leone is generally charged for and is provided by a mixture of government, private and non-governmental organizations (NGOs). There are over 100 NGOs operating in the health care sector in Sierra Leone. The Ministry of Health and Sanitation is responsible for organizing health care and after the end of the civil war the ministry changed to a decentralized structure of health provision to try to increase its coverage.
VigiBase is a World Health Organization's (WHO) global Individual Case Safety Report (ICSR) database that contains ICSRs submitted by the participating member states enrolled under WHO's international drug monitoring programme. It is the single largest drug safety data repository in the world. Since 1978, the Uppsala Monitoring Centre on behalf of WHO, have been maintaining VigiBase.

Voluntary Health Services, popularly known as the VHS Hospital, is a multispecialty tertiary care referral hospital in the south Indian state of Tamil Nadu, reportedly serving the economically weaker sections of the society. It was founded in 1958 by Krishnaswami Srinivas Sanjivi, an Indian physician, social worker and a winner of Padma Shri and Padma Bhushan awards and is run by a charitable non governmental organization of the same name. The hospital is situated along Rajiv Gandhi Salai at Taramani, in Chennai.
Trygg mammamedisin is a public service in Norway which gives individual advice about medical drugs to pregnant and breastfeeding women. The service is web-based, and is funded by the Ministry of Health and Care Services. Trygg mammamedisin is managed by RELIS - Regional Drug Information Centres. These centres are a publicly funded service for health professionals, offering producer-independent information about medical drugs. The answers provided in Trygg mammamedisin are generated by RELIS employees, who are all certified pharmacists or medical doctors working at the University Hospital of North Norway, Haukeland University Hospital, St. Olav's University Hospital and Oslo University Hospital.
Travel health nursing is a nursing specialty which promotes the health and safety of national and international travelers. Similar to travel medicine, it is an interdisciplinary practice which draws from the knowledge bases of vaccines, epidemiology, tropical medicine, public health, and health education. Travel nursing has experienced an increase in global demand due to the evolution of travel medicine. Travel health nursing was recognized during the 1980s as an emerging occupation to meet the needs of the traveling public, and additional education and training was established. Travel health nurses typically work in "private practice, hospital outpatient units, universities, the government, and the military", and have more opportunities and leadership roles as travel has become more common. However, they also experience organizational and support-related conflicts with general practitioners and patients in healthcare settings.






