
Methanol is an organic chemical compound and the simplest aliphatic alcohol, with the chemical formula CH3OH. It is a light, volatile, colorless and flammable liquid with a distinctive alcoholic odor similar to that of ethanol, but is more acutely toxic than the latter. Methanol acquired the name wood alcohol because it was once produced chiefly by the destructive distillation of wood. Today, methanol is mainly produced industrially by hydrogenation of carbon monoxide.
Syngas, or synthesis gas, is a mixture of hydrogen and carbon monoxide, in various ratios. The gas often contains some carbon dioxide and methane. It is principally used for producing ammonia or methanol. Syngas is combustible and can be used as a fuel. Historically, it has been used as a replacement for gasoline, when gasoline supply has been limited; for example, wood gas was used to power cars in Europe during WWII.

Methyl tert-butyl ether (MTBE), also known as tert-butyl methyl ether, is an organic compound with a structural formula (CH3)3COCH3. MTBE is a volatile, flammable, and colorless liquid that is sparingly soluble in water. Primarily used as a fuel additive, MTBE is blended into gasoline to increase its octane rating and knock resistance, and reduce unwanted emissions.
Methanol fuel is an alternative biofuel for internal combustion and other engines, either in combination with gasoline or independently. Methanol (CH3OH) is less expensive to sustainably produce than ethanol fuel, although it is more toxic than ethanol and has a lower energy density than gasoline. Methanol is safer for the environment than gasoline, is an anti-freeze agent, prevents dirt and grime buildup within the engine, has a higher ignition temperature and can withstand compression equivalent to that of super high-octane gasoline. It can readily be used in most modern engines. To prevent vapor lock due to being a simple, pure fuel, a small percentage of other fuel or certain additives can be included. Methanol may be made from fossil fuels or renewable resources, in particular natural gas and coal, or biomass respectively. In the case of the latter, it can be synthesized from CO2 (carbon dioxide) and hydrogen. The vast majority of methanol produced globally is currently made with gas and coal. However, projects, investments, and the production of green-methanol has risen steadily into 2023. Methanol fuel is currently used by racing cars in many countries and has seen increasing adoption by the maritime industry.

The hydrogen economy is an umbrella term for the roles hydrogen can play alongside low-carbon electricity to reduce emissions of greenhouse gases. The aim is to reduce emissions where cheaper and more energy-efficient clean solutions are not available. In this context, hydrogen economy encompasses the production of hydrogen and the use of hydrogen in ways that contribute to phasing-out fossil fuels and limiting climate change.

Direct methanol fuel cells or DMFCs are a subcategory of proton-exchange membrane fuel cells in which methanol is used as the fuel and a special proton-conducting polymer as the membrane (PEM). Their main advantage is low temperature operation and the ease of transport of methanol, an energy-dense yet reasonably stable liquid at all environmental conditions.
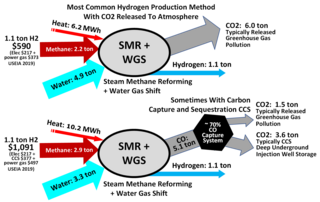
Steam reforming or steam methane reforming (SMR) is a method for producing syngas (hydrogen and carbon monoxide) by reaction of hydrocarbons with water. Commonly natural gas is the feedstock. The main purpose of this technology is often hydrogen production, although syngas has multiple other uses such as production of ammonia or methanol. The reaction is represented by this equilibrium:

Catalytic reforming is a chemical process used to convert naphthas from crude oil into liquid products called reformates, which are premium "blending stocks" for high-octane gasoline. The process converts low-octane linear hydrocarbons (paraffins) into branched alkanes (isoparaffins) and cyclic naphthenes, which are then partially dehydrogenated to produce high-octane aromatic hydrocarbons. The dehydrogenation also produces significant amounts of byproduct hydrogen gas, which is fed into other refinery processes such as hydrocracking. A side reaction is hydrogenolysis, which produces light hydrocarbons of lower value, such as methane, ethane, propane and butanes.

The methanol economy is a suggested future economy in which methanol and dimethyl ether replace fossil fuels as a means of energy storage, ground transportation fuel, and raw material for synthetic hydrocarbons and their products. It offers an alternative to the proposed hydrogen economy or ethanol economy, although these concepts are not exclusive. Methanol can be produced from a variety of sources including fossil fuels as well as agricultural products and municipal waste, wood and varied biomass. It can also be made from chemical recycling of carbon dioxide.

Gas to liquids (GTL) is a refinery process to convert natural gas or other gaseous hydrocarbons into longer-chain hydrocarbons, such as gasoline or diesel fuel. Methane-rich gases are converted into liquid synthetic fuels. Two general strategies exist: (i) direct partial combustion of methane to methanol and (ii) Fischer–Tropsch-like processes that convert carbon monoxide and hydrogen into hydrocarbons. Strategy ii is followed by diverse methods to convert the hydrogen-carbon monoxide mixtures to liquids. Direct partial combustion has been demonstrated in nature but not replicated commercially. Technologies reliant on partial combustion have been commercialized mainly in regions where natural gas is inexpensive.
Formic acid fuel cells (direct formic acid fuel cells or DFAFCs) are a subcategory of direct liquid-feed fuel cells (DLFCs), in which the liquid fuel is directly oxidized (electrochemically) at the anode instead of reforming to produce hydrogen. Formic acid-based fuel cells represent a promising energy supply system in terms of high volumetric energy density, theoretical energy efficiency, and theoretical open-circuit voltage. They are also able to overcome certain problems inherent to traditional hydrogen (H2) feed fuel cells such as safe handling, storage, and H2 transportation.

Various alcohols are used as fuel for internal combustion engines. The first four aliphatic alcohols are of interest as fuels because they can be synthesized chemically or biologically, and they have characteristics which allow them to be used in internal combustion engines. The general chemical formula for alcohol fuel is CnH2n+1OH.
Hydrogen technologies are technologies that relate to the production and use of hydrogen as a part hydrogen economy. Hydrogen technologies are applicable for many uses.
A methanol reformer is a device used in chemical engineering, especially in the area of fuel cell technology, which can produce pure hydrogen gas and carbon dioxide by reacting a methanol and water (steam) mixture.

Reformed Methanol Fuel Cell (RMFC) or Indirect Methanol Fuel Cell (IMFC) systems are a subcategory of proton-exchange fuel cells where, the fuel, methanol (CH3OH), is reformed, before being fed into the fuel cell.
The first time a catalyst was used in the industry was in 1746 by J. Roebuck in the manufacture of lead chamber sulfuric acid. Since then catalysts have been in use in a large portion of the chemical industry. In the start only pure components were used as catalysts, but after the year 1900 multicomponent catalysts were studied and are now commonly used in the industry.

Carbon Recycling International (CRI) is an Icelandic limited liability company which has developed a technology designed to produce renewable methanol, also known as e-methanol, from carbon dioxide and hydrogen, using water electrolysis or, alternatively, hydrogen captured from industrial waste gases. The technology is trademarked by CRI as Emissions-to-Liquids (ETL) and the renewable methanol produced by CRI is trademarked as Vulcanol. In 2011 CRI became the first company to produce and sell liquid renewable transport fuel produced using only carbon dioxide, water and electricity from renewable sources.
Green hydrogen (GH2 or GH2) is hydrogen produced by the electrolysis of water, using renewable electricity. Production of green hydrogen causes significantly lower greenhouse gas emissions than production of grey hydrogen, which is derived from fossil fuels without carbon capture.

The Gumpert Nathalie or RG Nathalie is a hydrogen-electric hybrid sports car running on methanol to generate hydrogen that is scheduled to enter production in 2021. It is the first car produced by the new car manufacturer RG founded by Roland Gumpert, following the bankruptcy of his company Gumpert which produced the Apollo. Production is planned to be limited to 500 cars.
High Temperature Proton Exchange Membrane fuel cells (HT-PEMFC), also known as High Temperature Polymer Electrolyte Membrane fuel cells, are a type of PEM fuel cells which can be operated at temperatures between 120 and 200°C. HT-PEM fuel cells are used for both stationary and portable applications. The HT-PEM fuel cell is usually supplied with hydrogen-rich gas like reformate gas formed by reforming of methanol, ethanol, natural gas or LPG.











