Related Research Articles

In cell biology a centriole is a cylindrical organelle composed mainly of a protein called tubulin. Centrioles are found in most eukaryotic cells, but are not present in conifers (Pinophyta), flowering plants (angiosperms) and most fungi, and are only present in the male gametes of charophytes, bryophytes, seedless vascular plants, cycads, and Ginkgo. A bound pair of centrioles, surrounded by a highly ordered mass of dense material, called the pericentriolar material (PCM), makes up a structure called a centrosome.

Microtubules are polymers of tubulin that form part of the cytoskeleton and provide structure and shape to eukaryotic cells. Microtubules can be as long as 50 micrometres, as wide as 23 to 27 nm and have an inner diameter between 11 and 15 nm. They are formed by the polymerization of a dimer of two globular proteins, alpha and beta tubulin into protofilaments that can then associate laterally to form a hollow tube, the microtubule. The most common form of a microtubule consists of 13 protofilaments in the tubular arrangement.

In cell biology, the centrosome is an organelle that serves as the main microtubule organizing center (MTOC) of the animal cell, as well as a regulator of cell-cycle progression. The centrosome provides structure for the cell. The centrosome is thought to have evolved only in the metazoan lineage of eukaryotic cells. Fungi and plants lack centrosomes and therefore use other structures to organize their microtubules. Although the centrosome has a key role in efficient mitosis in animal cells, it is not essential in certain fly and flatworm species.
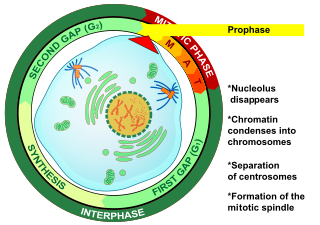
Prophase is the first stage of cell division in both mitosis and meiosis. Beginning after interphase, DNA has already been replicated when the cell enters prophase. The main occurrences in prophase are the condensation of the chromatin reticulum and the disappearance of the nucleolus.

A basal body is a protein structure found at the base of a eukaryotic undulipodium. The basal body was named by Theodor Wilhelm Engelmann in 1880. It is formed from a centriole and several additional protein structures, and is, essentially, a modified centriole. The basal body serves as a nucleation site for the growth of the axoneme microtubules. Centrioles, from which basal bodies are derived, act as anchoring sites for proteins that in turn anchor microtubules, and are known as the microtubule organizing center (MTOC). These microtubules provide structure and facilitate movement of vesicles and organelles within many eukaryotic cells.
David Moore Glover is a British geneticist and Research Professor of Biology and Biological Engineering at the California Institute of Technology. He served as Balfour Professor of Genetics at the University of Cambridge, a Wellcome Trust investigator in the Department of Genetics at the University of Cambridge, and Fellow of Fitzwilliam College, Cambridge. He serves as the first editor-in-chief of the open-access journal Open Biology published by the Royal Society.
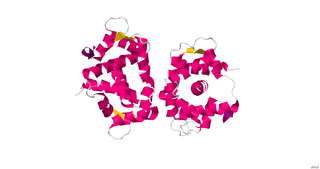
Centrins, also known as caltractins, are a family of calcium-binding phosphoproteins found in the centrosome of eukaryotes. Centrins are small calcium binding proteins that are ubiquitous centrosome components. There are about 350 “signature” proteins that are unique to eukaryotic cells but have no significant homology to proteins in archaea and bacteria. They are a type of protein that is essential and present in almost all eukaryotic cells and are found in the centrioles and pericentriolar lattice. Human centrin genes are CETN1, CETN2 and CETN3.

Pericentrin (kendrin), also known as PCNT and pericentrin-B (PCNTB), is a protein which in humans is encoded by the PCNT gene on chromosome 21. This protein localizes to the centrosome and recruits proteins to the pericentriolar matrix (PCM) to ensure proper centrosome and mitotic spindle formation, and thus, uninterrupted cell cycle progression. This gene is implicated in many diseases and disorders, including congenital disorders such as microcephalic osteodysplastic primordial dwarfism type II (MOPDII) and Seckel syndrome.
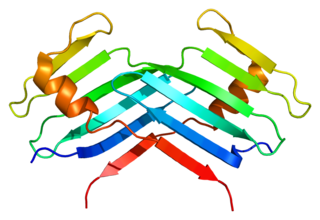
Serine/threonine-protein kinase PLK4 also known as polo-like kinase 4 is an enzyme that in humans is encoded by the PLK4 gene. The Drosophila homolog is SAK, the C elegans homolog is zyg-1, and the Xenopus homolog is Plx4.

Centrosomal protein 170kDa, also known as CEP170, is a protein that in humans is encoded by the CEP170 gene.

Pericentriolar material is a highly structured, dense mass of protein which makes up the part of the animal centrosome that surrounds the two centrioles. The PCM contains proteins responsible for microtubule nucleation and anchoring including γ-tubulin, pericentrin and ninein.

Spindle assembly abnormal protein 6 homolog (SAS-6) is a protein that in humans is encoded by the SASS6 gene.

Centrosomes are the major microtubule organizing centers (MTOC) in mammalian cells. Failure of centrosome regulation can cause mistakes in chromosome segregation and is associated with aneuploidy. A centrosome is composed of two orthogonal cylindrical protein assemblies, called centrioles, which are surrounded by a protein dense amorphous cloud of pericentriolar material (PCM). The PCM is essential for nucleation and organization of microtubules. The centrosome cycle is important to ensure that daughter cells receive a centrosome after cell division. As the cell cycle progresses, the centrosome undergoes a series of morphological and functional changes. Initiation of the centrosome cycle occurs early in the cell cycle in order to have two centrosomes by the time mitosis occurs.
Gillian Griffiths, FMedSci FRS is a British cell biologist and immunologist. Griffiths was one of the first to show that immune cells have specialised mechanisms of secretion, and identified proteins and mechanisms that control cytotoxic T lymphocyte secretion. Griffiths is Professor of Cell Biology and Immunology at the University of Cambridge and is the Director of the Cambridge Institute for Medical Research.
Mónica Bettencourt-Dias is a Portuguese biochemist and cellular biologist, who is the head of the Cell Cycle Regulation research group at the Instituto Gulbenkian de Ciência. Her research involves cell cycle regulation, for which she has been recognized as the recipient of the Pfizer Award for Basic Research, the Keith Porter Prize from the American Society for Cell Biology and the Eppendorf Young European Investigator Award. She was also selected as a 2009 European Molecular Biology Organization Young Investigator Fellow and inducted as a member of the EMBO in 2015. Mónica Bettencourt-Dias was appointed Director of Instituto Gulbenkian de Ciência in November, 2017.
Karen Oegema is a molecular cell biologist at the Ludwig Institute for Cancer Research and a professor of cellular and molecular medicine at the University of California, San Diego. She is best known for her research with Caenorhabditis elegans, which her lab uses as a model system in their mission to dissect the molecular mechanics of cytokinesis. She was given the Women in Cell Biology Mid-Career Award for Excellence in Research in 2017, as well as the Women in Cell Biology Junior Award for Excellence in Research in 2006.

Melina Schuh is a German biochemist and Director at the Max Planck Institute for Multidisciplinary Sciences. She is known for her work on meiosis in mammalian oocytes, for her studies on the mechanisms leading to the age-related decline in female fertility, and for the development of the Trim-Away protein depletion method.

Sharon Tooze, FMedSci is an American cell biologist who has made significant contributions to the Autophagy field. She is a senior scientist at the Francis Crick Institute and was awarded European Molecular Biology Organization (EMBO) membership in 2010.

Pierre Gönczy is a Swiss and Italian cell and developmental biologist. His research focuses on centriole biology and asymmetric cell division. He is currently professor at École Polytechnique Fédérale de Lausanne (EPFL), where he directs the Laboratory of Cell and Developmental Biology.
Ludger Johannes is a French-German biochemist who has specialized in the field of endocytosis and intracellular trafficking. He and his team study how sugars attached to proteins or lipids influence the transport of biological material into eukaryotic cells.
References
- 1 2 "Cell dynamics research: Institut Curie, subcellular structure and cellular dynamics". Centre de recherche de l'Institut Curie. Retrieved 2020-04-16.
- 1 2 "Renata Homem de Gouveia Xavier de BASTO". www.barrosbrito.com. Retrieved 2020-04-17.
- 1 2 "Renata Basto | INSU". www.insu.cnrs.fr. Retrieved 2020-04-17.
- ↑ "Renata Homem de Gouveia Xavier de Basto". arquivo.fct.pt. Retrieved 2020-04-17.
- ↑ "Previous Lab Members | Raff Lab". www2.bioch.ox.ac.uk. Retrieved 2020-04-16.
- ↑ Basto, Renata; Gomes, Rui; Karess, Roger E. (December 2000). "Rough Deal and Zw10 are required for the metaphase checkpoint in Drosophila". Nature Cell Biology. 2 (12): 939–943. doi:10.1038/35046592. ISSN 1476-4679. PMID 11146659. S2CID 27007621.
- ↑ Basto, Renata; Lau, Joyce; Vinogradova, Tatiana; Gardiol, Alejandra; Woods, C. Geoffrey; Khodjakov, Alexey; Raff, Jordan W. (2006-06-30). "Flies without Centrioles". Cell. 125 (7): 1375–1386. doi: 10.1016/j.cell.2006.05.025 . ISSN 0092-8674. PMID 16814722. S2CID 2080684.
- ↑ Basto, Renata; Brunk, Kathrin; Vinadogrova, Tatiana; Peel, Nina; Franz, Anna; Khodjakov, Alexey; Raff, Jordan W. (2008-06-13). "Centrosome Amplification Can Initiate Tumorigenesis in Flies". Cell. 133 (6): 1032–1042. doi:10.1016/j.cell.2008.05.039. ISSN 0092-8674. PMC 2653712 . PMID 18555779.
- ↑ "Biology of centrosomes and genetic instability". Centre de recherche de l'Institut Curie. Retrieved 2020-04-16.
- ↑ Marthiens, Véronique; Rujano, Maria A.; Pennetier, Carole; Tessier, Sarah; Paul-Gilloteaux, Perrine; Basto, Renata (2013-05-12). "Centrosome amplification causes microcephaly". Nature Cell Biology. 15 (7): 731–740. doi:10.1038/ncb2746. ISSN 1465-7392. PMID 23666084. S2CID 20319825.
- ↑ Gambarotto, Davide; Pennetier, Carole; Ryniawec, John M.; Buster, Daniel W.; Gogendeau, Delphine; Goupil, Alix; Nano, Maddalena; Simon, Anthony; Blanc, Damien; Racine, Victor; Kimata, Yuu (2019-07-01). "Plk4 Regulates Centriole Asymmetry and Spindle Orientation in Neural Stem Cells". Developmental Cell. 50 (1): 11–24.e10. doi: 10.1016/j.devcel.2019.04.036 . ISSN 1534-5807. PMC 6614718 . PMID 31130353.
- ↑ Gogendeau, Delphine; Siudeja, Katarzyna; Gambarotto, Davide; Pennetier, Carole; Bardin, Allison J.; Basto, Renata (2015-11-17). "Aneuploidy causes premature differentiation of neural and intestinal stem cells". Nature Communications. 6 (1): 8894. doi: 10.1038/ncomms9894 . ISSN 2041-1723. PMC 4660207 . PMID 26573328.
- ↑ Nano, Maddalena; Gemble, Simon; Simon, Anthony; Pennetier, Carole; Fraisier, Vincent; Marthiens, Veronique; Basto, Renata (2019-11-18). "Cell-Cycle Asynchrony Generates DNA Damage at Mitotic Entry in Polyploid Cells". Current Biology. 29 (22): 3937–3945.e7. doi: 10.1016/j.cub.2019.09.041 . ISSN 0960-9822. PMID 31708395. S2CID 207952376.
- ↑ Goupil, Alix; Nano, Maddalena; Letort, Gaëlle; Gemble, Simon; Edwards, Frances; Goundiam, Oumou; Gogendeau, Delphine; Pennetier, Carole; Basto, Renata (2020-04-06). "Chromosomes function as a barrier to mitotic spindle bipolarity in polyploid cells". Journal of Cell Biology. 219 (4). doi: 10.1083/jcb.201908006 . ISSN 0021-9525. PMC 7147111 . PMID 32328633.
- ↑ Methods in Cilia and Flagella. Academic Press. 2015-03-26. ISBN 978-0-12-802640-3.
- ↑ "Renata Basto | Semantic Scholar". www.semanticscholar.org. Retrieved 2020-04-17.
- 1 2 "ERC FUNDED PROJECTS". ERC: European Research Council. Archived from the original on 2021-01-13. Retrieved 2020-04-17.
- ↑ "EU research".