A research site is a place where people conduct research. Common research sites include universities, hospitals, research institutes, and field research locations.
A research site is a place where people conduct research. Common research sites include universities, hospitals, research institutes, and field research locations.
In clinical research a research site conducts all or part of a clinical trial. For clinical trials which recruit research participants in multiple locations, often the research will have a headquarters then multiple regional research sites to conduct the research in that region. In a network of research sites where all are recruiting study participants, sites with low recruitment benefit from coaching from sites with high recruitment. [1]
Characteristics of good clinical research sites include setting good timelines, early participant recruitment, and having a management plan for efficiency. [2]
Researchers in nursing have reported challenges accessing the facilities designated for conventional medical research. [3]
The design of a research site should have a means of detecting fraud. [4]
Researchers who do not have a cultural tie to a research population may have difficulty doing ethnographic research with that community. [5]

A randomized controlled trial is a form of scientific experiment used to control factors not under direct experimental control. Examples of RCTs are clinical trials that compare the effects of drugs, surgical techniques, medical devices, diagnostic procedures or other medical treatments.

Clinical trials are prospective biomedical or behavioral research studies on human participants designed to answer specific questions about biomedical or behavioral interventions, including new treatments and known interventions that warrant further study and comparison. Clinical trials generate data on dosage, safety and efficacy. They are conducted only after they have received health authority/ethics committee approval in the country where approval of the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial—their approval does not mean the therapy is 'safe' or effective, only that the trial may be conducted.

Human subject research is systematic, scientific investigation that can be either interventional or observational and involves human beings as research subjects, commonly known as test subjects. Human subject research can be either medical (clinical) research or non-medical research. Systematic investigation incorporates both the collection and analysis of data in order to answer a specific question. Medical human subject research often involves analysis of biological specimens, epidemiological and behavioral studies and medical chart review studies. On the other hand, human subject research in the social sciences often involves surveys which consist of questions to a particular group of people. Survey methodology includes questionnaires, interviews, and focus groups.
Observer bias is one of the types of detection bias and is defined as any kind of systematic divergence from accurate facts during observation and the recording of data and information in studies. The definition can be further expanded upon to include the systematic difference between what is observed due to variation in observers, and what the true value is.
An adverse event (AE) is any untoward medical occurrence in a patient or clinical investigation subject administered a pharmaceutical product and which does not necessarily have a causal relationship with this treatment. An adverse event can therefore be any unfavourable and unintended sign, symptom, or disease temporally associated with the use of a medicinal (investigational) product, whether or not related to the medicinal (investigational) product.
The Declaration of Helsinki is a set of ethical principles regarding human experimentation developed originally in 1964 for the medical community by the World Medical Association (WMA). It is widely regarded as the cornerstone document on human research ethics.
A pilot experiment, pilot study, pilot test or pilot project is a small-scale preliminary study conducted to evaluate feasibility, duration, cost, adverse events, and improve upon the study design prior to performance of a full-scale research project.
A Clinical Trial Management System (CTMS) is a software system used by biotechnology and pharmaceutical industries to manage clinical trials in clinical research. The system maintains and manages planning, performing and reporting functions, along with participant contact information, tracking deadlines and milestones.

PatientsLikeMe is an integrated community, health management, and real-world data platform. The platform currently has over 830,000 members who are dealing with more than 2,900 conditions. Data generated by patients themselves are systemically collected and quantified with the goal of providing an environment for peer support and learning. These data capture the complex temporality and competing influences of different lifestyle choices, socio-demographics, conditions and treatments on a person's health. While striving to empower the community with personal agency, PLM has also established itself as a clinical resource with more than 100 studies in peer-reviewed medical and scientific journals.

Oncology is a branch of medicine that deals with the study, treatment, diagnosis and prevention of cancer. A medical professional who practices oncology is an oncologist. The name's etymological origin is the Greek word ὄγκος (ónkos), meaning "tumor", "volume" or "mass". Oncology is concerned with:
Female genital disease is a disorder of the structure or function of the female reproductive system that has a known cause and a distinctive group of symptoms, signs, or anatomical changes. The female reproductive system consists of the ovaries, fallopian tubes, uterus, vagina, and vulva. Female genital diseases can be classified by affected location or by type of disease, such as malformation, inflammation, or infection.
Patient participation is a trend that arose in answer to medical paternalism. Informed consent is a process where patients make decisions informed by the advice of medical professionals.
Patient recruitment includes a variety of services—typically performed by a Patient Recruitment Service Provider—to increase enrollment into clinical trials. Presently, the patient recruitment industry is claimed to total $19 billion per year.
Parkinson's disease clinical research is any study intended to help answer questions about etiology, diagnostic approaches or new treatments by studying their effects on human subjects. Clinical trials are designed and conducted by scientists and medical experts, who invite participants to undergo testing new vaccines, therapies, or treatments.

The phases of clinical research are the stages in which scientists conduct experiments with a health intervention to obtain sufficient evidence for a process considered effective as a medical treatment. For drug development, the clinical phases start with testing for drug safety in a few human subjects, then expand to many study participants to determine if the treatment is effective. Clinical research is conducted on drug candidates, vaccine candidates, new medical devices, and new diagnostic assays.
Patient satisfaction is a measure of the extent to which a patient is content with the health care which they received from their health care provider.

OpenNotes is a research initiative and international movement located at Beth Israel Deaconess Medical Center.
A human challenge study, also called a challenge trial or controlled human infection model (CHIM), is a type of clinical trial for a vaccine or other pharmaceutical involving the intentional exposure of the test subject to the condition tested. Human challenge studies may be ethically controversial because they involve exposing test subjects to dangers beyond those posed by potential side effects of the substance being tested.

Clinical trials in India refers to clinical research in India in which researchers test drugs and other treatments on research participants. NDCTR 2019 and section 3.7.1 to 3.7.3 of ICMR guidelines requires that all researchers conducting a clinical trial must publicly document it in the Clinical Trials Registry - India.
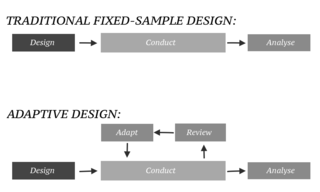
In an adaptive design of a clinical trial, the parameters and conduct of the trial for a candidate drug or vaccine may be changed based on an interim analysis. Adaptive design typically involves advanced statistics to interpret a clinical trial endpoint. This is in contrast to traditional single-arm clinical trials or randomized clinical trials (RCTs) that are static in their protocol and do not modify any parameters until the trial is completed. The adaptation process takes place at certain points in the trial, prescribed in the trial protocol. Importantly, this trial protocol is set before the trial begins with the adaptation schedule and processes specified. Adaptions may include modifications to: dosage, sample size, drug undergoing trial, patient selection criteria and/or "cocktail" mix. The PANDA provides not only a summary of different adaptive designs, but also comprehensive information on adaptive design planning, conduct, analysis and reporting.