
A perovskite is any material with a crystal structure following the formula ABX3, which was first discovered as the mineral called perovskite, which consists of calcium titanium oxide (CaTiO3). The mineral was first discovered in the Ural mountains of Russia by Gustav Rose in 1839 and named after Russian mineralogist L. A. Perovski (1792–1856). 'A' and 'B' are two positively charged ions (i.e. cations), often of very different sizes, and X is a negatively charged ion (an anion, frequently oxide) that bonds to both cations. The 'A' atoms are generally larger than the 'B' atoms. The ideal cubic structure has the B cation in 6-fold coordination, surrounded by an octahedron of anions, and the A cation in 12-fold cuboctahedral coordination. Additional perovskite forms may exist where either/both the A and B sites have a configuration of A1x-1A2x and/or B1y-1B2y and the X may deviate from the ideal coordination configuration as ions within the A and B sites undergo changes in their oxidation states.
Organic semiconductors are solids whose building blocks are pi-bonded molecules or polymers made up by carbon and hydrogen atoms and – at times – heteroatoms such as nitrogen, sulfur and oxygen. They exist in the form of molecular crystals or amorphous thin films. In general, they are electrical insulators, but become semiconducting when charges are either injected from appropriate electrodes, upon doping or by photoexcitation.
Hybrid solar cells combine advantages of both organic and inorganic semiconductors. Hybrid photovoltaics have organic materials that consist of conjugated polymers that absorb light as the donor and transport holes. Inorganic materials in hybrid cells are used as the acceptor and electron transporter in the structure. The hybrid photovoltaic devices have a potential for not only low-cost by roll-to-roll processing but also for scalable solar power conversion.
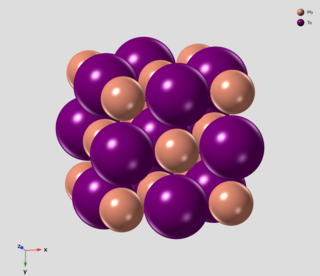
Lead telluride is a compound of lead and tellurium (PbTe). It crystallizes in the NaCl crystal structure with Pb atoms occupying the cation and Te forming the anionic lattice. It is a narrow gap semiconductor with a band gap of 0.32 eV. It occurs naturally as the mineral altaite.
A chalcogel or properly metal chalcogenide aerogel is an aerogel made from chalcogenides. Chalcogels preferentially absorb heavy metals, such as mercury, lead, and cadmium, from water. Sulfide chalcogels are also very good at desulfurization.

A biointerface is the region of contact between a biomolecule, cell, biological tissue or living organism or organic material considered living with another biomaterial or inorganic/organic material. The motivation for biointerface science stems from the urgent need to increase the understanding of interactions between biomolecules and surfaces. The behavior of complex macromolecular systems at materials interfaces are important in the fields of biology, biotechnology, diagnostics, and medicine. Biointerface science is a multidisciplinary field in which biochemists who synthesize novel classes of biomolecules cooperate with scientists who have developed the tools to position biomolecules with molecular precision, scientists who have developed new spectroscopic techniques to interrogate these molecules at the solid-liquid interface, and people who integrate these into functional devices. Well-designed biointerfaces would facilitate desirable interactions by providing optimized surfaces where biological matter can interact with other inorganic or organic materials, such as by promoting cell and tissue adhesion onto a surface.
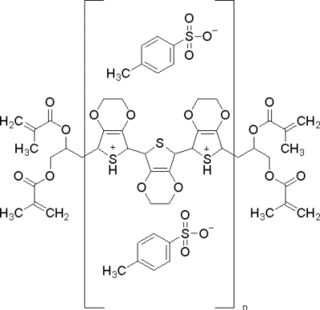
Poly(3,4-ethylenedioxythiophene)-tetramethacrylate or PEDOT-TMA is a p-type conducting polymer based on 3,4-ethylenedioxylthiophene or the EDOT monomer. It is a modification of the PEDOT structure. Advantages of this polymer relative to PEDOT are that it is dispersible in organic solvents, and it is non-corrosive. PEDOT-TMA was developed under a contract with the National Science Foundation, and it was first announced publicly on April 12, 2004. The trade name for PEDOT-TMA is Oligotron. PEDOT-TMA was featured in an article entitled "Next Stretch for Plastic Electronics" that appeared in Scientific American in 2004. The U.S. Patent office issued a patent protecting PEDOT-TMA on April 22, 2008.
Organic photovoltaic devices (OPVs) are fabricated from thin films of organic semiconductors, such as polymers and small-molecule compounds, and are typically on the order of 100 nm thick. Because polymer based OPVs can be made using a coating process such as spin coating or inkjet printing, they are an attractive option for inexpensively covering large areas as well as flexible plastic surfaces. A promising low cost alternative to conventional solar cells made of crystalline silicon, there is a large amount of research being dedicated throughout industry and academia towards developing OPVs and increasing their power conversion efficiency.
An organic solar cell (OSC) or plastic solar cell is a type of photovoltaic that uses organic electronics, a branch of electronics that deals with conductive organic polymers or small organic molecules, for light absorption and charge transport to produce electricity from sunlight by the photovoltaic effect. Most organic photovoltaic cells are polymer solar cells.

Crystalline silicon or (c-Si) Is the crystalline forms of silicon, either polycrystalline silicon, or monocrystalline silicon. Crystalline silicon is the dominant semiconducting material used in photovoltaic technology for the production of solar cells. These cells are assembled into solar panels as part of a photovoltaic system to generate solar power from sunlight.
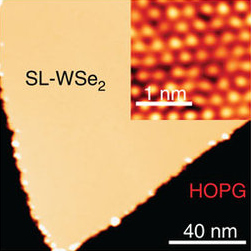
Tungsten diselenide is an inorganic compound with the formula WSe2. The compound adopts a hexagonal crystalline structure similar to molybdenum disulfide. The tungsten atoms are covalently bonded to six selenium ligands in a trigonal prismatic coordination sphere while each selenium is bonded to three tungsten atoms in a pyramidal geometry. The tungsten–selenium bond has a length of 0.2526 nm, and the distance between selenium atoms is 0.334 nm. It is a well studied example of a layered material. The layers stack together via van der Waals interactions. WSe2 is a very stable semiconductor in the group-VI transition metal dichalcogenides.

A perovskite solar cell (PSC) is a type of solar cell that includes a perovskite-structured compound, most commonly a hybrid organic–inorganic lead or tin halide-based material as the light-harvesting active layer. Perovskite materials, such as methylammonium lead halides and all-inorganic cesium lead halide, are cheap to produce and simple to manufacture.

Polymer-fullerene bulk heterojunction solar cells are a type of solar cell researched in academic laboratories. Photovoltaic cells featuring a polymeric blend of organics have shown promise in a field largely dominated by inorganic solar cells. Specifically, fullerene derivatives act as electron acceptors for donor materials like P3HT, creating a polymer-fullerene based photovoltaic cell.

In a basic Schottky-junction (Schottky-barrier) solar cell, an interface between a metal and a semiconductor provides the band bending necessary for charge separation. Traditional solar cells are composed of p-type and n-type semiconductor layers sandwiched together, forming the source of built-in voltage. Due to differing energy levels between the Fermi level of the metal and the conduction band of the semiconductor, an abrupt potential difference is created, instead of the smooth band transition observed across a p-n junction in a standard solar cell, and this is a Schottky height barrier. Although vulnerable to higher rates of thermionic emission, manufacturing of Schottky barrier solar cells proves to be cost-effective and industrially scalable.
A tin-based perovskite solar cell is a special type of perovskite solar cell, where the lead is substituted by tin. It has a tin-based perovskite structure (ASnX3), where 'A' is a 1+ cation and 'X' is a monovalent halogen anion. The methylammonium tin triiodide (CH3NH3SnI3) has a band gap of 1.2–1.3 eV, while formamidinium tin triiodide has a band gap of 1.4 eV.

Mercouri Kanatzidis is a Charles E. and Emma H. Morrison Professor of chemistry and professor of materials science and engineering at Northwestern University and Senior Scientist at Argonne National Laboratory.

Perovskite nanocrystals are a class of semiconductor nanocrystals, which exhibit unique characteristics that separate them from traditional quantum dots. Perovskite nanocrystals have an ABX3 composition where A = cesium, methylammonium (MA), or formamidinium (FA); B = lead or tin; and X = chloride, bromide, or iodide.
André Taylor is an American scientist who is an associate professor of chemical engineering at the New York University Tandon School of Engineering. Taylor works on novel materials for energy conversion and storage. He was awarded the Presidential Early Career Award for Scientists and Engineers in 2010, and named as one of The Community of Scholars' Most Influential Black Researchers of 2020.
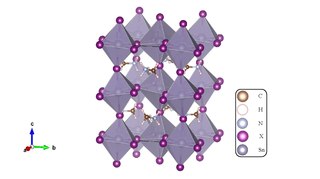
Methylammonium tin halides are solid compounds with perovskite structure and a chemical formula of CH3NH3SnX3, where X = I, Br or Cl. They are promising lead-free alternatives to lead perovskites as photoactive semiconductor materials. Tin-based perovskites have shown excellent mobility in transistors which gives them an opportunity to be explored more for solar cell applications.
Kyoung-Shin Choi is a professor of chemistry at the University of Wisconsin-Madison. Choi's research focuses on the electrochemical synthesis of electrode materials, for use in electrochemical and photoelectrochemical devices.













