Related Research Articles

The serotonin transporter also known as the sodium-dependent serotonin transporter and solute carrier family 6 member 4 is a protein that in humans is encoded by the SLC6A4 gene. SERT is a type of monoamine transporter protein that transports the neurotransmitter serotonin from the synaptic cleft back to the presynaptic neuron, in a process known as serotonin reuptake.
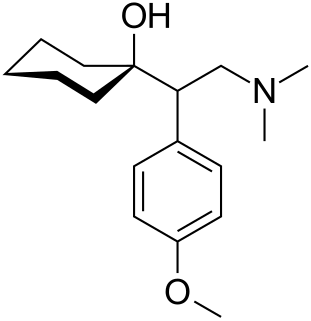
Venlafaxine, sold under the brand name Effexor among others, is an antidepressant medication of the serotonin-norepinephrine reuptake inhibitor (SNRI) class. It is used to treat major depressive disorder (MDD), generalized anxiety disorder (GAD), panic disorder, and social anxiety disorder (SAD). It may also be used for chronic pain. It is taken by mouth. It is also available as the salt venlafaxine besylate in an extended-release formulation.

Catechol-O-methyltransferase is one of several enzymes that degrade catecholamines, catecholestrogens, and various drugs and substances having a catechol structure. In humans, catechol-O-methyltransferase protein is encoded by the COMT gene. Two isoforms of COMT are produced: the soluble short form (S-COMT) and the membrane bound long form (MB-COMT). As the regulation of catecholamines is impaired in a number of medical conditions, several pharmaceutical drugs target COMT to alter its activity and therefore the availability of catecholamines. COMT was first discovered by the biochemist Julius Axelrod in 1957.

The norepinephrine transporter (NET), also known as noradrenaline transporter (NAT), is a protein that in humans is encoded by the solute carrier family 6 member 2 (SLC6A2) gene.

The 5-HT2A receptor is a subtype of the 5-HT2 receptor that belongs to the serotonin receptor family and is a G protein-coupled receptor (GPCR). The 5-HT2A receptor is a cell surface receptor, but has several intracellular locations. 5-HT is short for 5-hydroxy-tryptamine or serotonin. This is the main excitatory receptor subtype among the GPCRs for serotonin, although 5-HT2A may also have an inhibitory effect on certain areas such as the visual cortex and the orbitofrontal cortex. This receptor was first noted for its importance as a target of serotonergic psychedelic drugs such as LSD and psilocybin mushrooms. Later it came back to prominence because it was also found to be mediating, at least partly, the action of many antipsychotic drugs, especially the atypical ones.

Cytochrome P450 2B6 is an enzyme that in humans is encoded by the CYP2B6 gene. CYP2B6 is a member of the cytochrome P450 group of enzymes. Along with CYP2A6, it is involved with metabolizing nicotine, along with many other substances.

The serotonin 1A receptor is a subtype of serotonin receptor, or 5-HT receptor, that binds serotonin, also known as 5-HT, a neurotransmitter. 5-HT1A is expressed in the brain, spleen, and neonatal kidney. It is a G protein-coupled receptor (GPCR), coupled to the Gi protein, and its activation in the brain mediates hyperpolarisation and reduction of firing rate of the postsynaptic neuron. In humans, the serotonin 1A receptor is encoded by the HTR1A gene.

Glutamate receptor, ionotropic, kainate 1, also known as GRIK1, is a protein that in humans is encoded by the GRIK1 gene.

Glutamate receptor, ionotropic kainate 3 is a protein that in humans is encoded by the GRIK3 gene.
rs6295, also called C(-1019)G, is a gene variation—a single nucleotide polymorphism (SNP)—in the HTR1A gene. It is one of the most investigated SNPs of its gene. The C-allele is the most prevalent with 0.675 against the G-allele with 0.325 among Caucasian.
5-HTTLPR is a degenerate repeat polymorphic region in SLC6A4, the gene that codes for the serotonin transporter. Since the polymorphism was identified in the middle of the 1990s, it has been extensively investigated, e.g., in connection with neuropsychiatric disorders. A 2006 scientific article stated that "over 300 behavioral, psychiatric, pharmacogenetic and other medical genetics papers" had analyzed the polymorphism. While often discussed as an example of gene-environment interaction, this contention is contested.
In genetics, rs7997012 is a gene variation—a single nucleotide polymorphism (SNP)—in intron 2 of the human HTR2A gene that codes for the 5-HT2A receptor. The SNP varies between adenine (A) and guanine (G) DNA bases with the G-allele being most frequent. A research study found it to be related to antidepressant treatment. The research group reported that a polymorphism (rs1954787) on another gene, the GRIK4, has also shown a treatment-response-association in this kind of treatment. In a Japanese study rs7997012 was not associated with either major depressive disorder or bipolar disorder.

GRIK4 is a kainate receptor subtype belonging to the family of ligand-gated ion channels which is encoded by the GRIK4 gene.
In genetics, Rs1805054, also called C267T, is a name used for a specific genetic variation, a single nucleotide polymorphism (SNP), in the HTR6 gene. It is one of the few investigated polymorphisms of its gene. C267T is a synonymous polymorphism.

5-hydroxytryptamine (serotonin) receptor 3B, also known as HTR3B, is a human gene. The protein encoded by this gene is a subunit of the 5-HT3 receptor.
Scientific studies have found that different brain areas show altered activity in people with major depressive disorder (MDD), and this has encouraged advocates of various theories that seek to identify a biochemical origin of the disease, as opposed to theories that emphasize psychological or situational causes. Factors spanning these causative groups include nutritional deficiencies in magnesium, vitamin D, and tryptophan with situational origin but biological impact. Several theories concerning the biologically based cause of depression have been suggested over the years, including theories revolving around monoamine neurotransmitters, neuroplasticity, neurogenesis, inflammation and the circadian rhythm. Physical illnesses, including hypothyroidism and mitochondrial disease, can also trigger depressive symptoms.
The pharmacology of antidepressants is not entirely clear. The earliest and probably most widely accepted scientific theory of antidepressant action is the monoamine hypothesis, which states that depression is due to an imbalance of the monoamine neurotransmitters. It was originally proposed based on the observation that certain hydrazine anti-tuberculosis agents produce antidepressant effects, which was later linked to their inhibitory effects on monoamine oxidase, the enzyme that catalyses the breakdown of the monoamine neurotransmitters. All currently marketed antidepressants have the monoamine hypothesis as their theoretical basis, with the possible exception of agomelatine which acts on a dual melatonergic-serotonergic pathway. Despite the success of the monoamine hypothesis it has a number of limitations: for one, all monoaminergic antidepressants have a delayed onset of action of at least a week; and secondly, there are a sizeable portion (>40%) of depressed patients that do not adequately respond to monoaminergic antidepressants. Further evidence to the contrary of the monoamine hypothesis are the recent findings that a single intravenous infusion with ketamine, an antagonist of the NMDA receptor — a type of glutamate receptor — produces rapid, robust and sustained antidepressant effects. Monoamine precursor depletion also fails to alter mood. To overcome these flaws with the monoamine hypothesis a number of alternative hypotheses have been proposed, including the glutamate, neurogenic, epigenetic, cortisol hypersecretion and inflammatory hypotheses. Another hypothesis that has been proposed which would explain the delay is the hypothesis that monoamines don't directly influence mood, but influence emotional perception biases.
Epigenetics of depression is the study of how epigenetics contribute to depression.
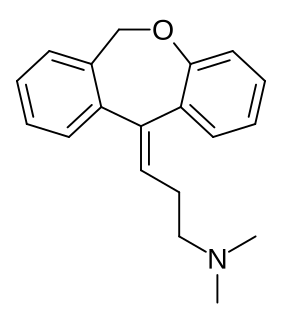
Cidoxepin (former developmental code name P-4599), also known as cis-doxepin or (Z)-doxepin, is a tricyclic antidepressant which was developed in the 1960s but was never marketed. It is the cis or (Z) stereoisomer of doxepin, a mixture of (E) and (Z) isomers that is used commercially in a ratio of approximately 85:15 with cidoxepin as a relatively minor constituent. However, the drug has similar activity to that of doxepin, acting as a serotonin–norepinephrine reuptake inhibitor, H1 receptor antagonist, and anticholinergic, and notably is thought to have more antidepressant activity than trans-doxepin. The central anticholinergic activity of cidoxepin has been reported to be 3-fold greater than that of the trans isomer in mice.
TLQP-62 (amino acid 556-617) is a VGF-derived C-terminal peptide that was first discovered by Trani et al. TLQP-62 is derived from VGF precursor protein via proteolytic cleavage by prohormone convertases PC1/3 at the RPR555 site. TLQP-62 is named after its first four N-terminal amino acids and its peptide length.
References
- ↑ Silvia Paddock, Gonzalo Laje, Dennis Charney, A. John Rush, Alexander F. Wilson, Alexa J.M. Sorant, Robert Lipsky, Stephen R. Wisniewski, Husseini Manji and Francis J. McMahon (August 2007). "Association of GRIK4 With Outcome of Antidepressant Treatment in the STAR*D Cohort". Am J Psychiatry . 164 (8): 1181–1188. doi:10.1176/appi.ajp.2007.06111790. PMID 17671280. S2CID 13306769.
{{cite journal}}: CS1 maint: multiple names: authors list (link)