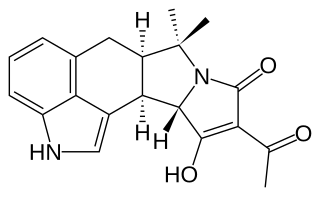
Cyclopiazonic acid (CPA) is a toxic fungal secondary metabolite. Chemically, CPA is related to ergoline alkaloids. CPA was originally isolated from Penicillium cyclopium and subsequently from other fungi including Penicillium griseofulvum, Penicillium camemberti, Penicillium commune, Aspergillus flavus, and Aspergillus versicolor. CPA only appears to be toxic in high concentrations. Biologically, CPA is a specific inhibitor of SERCA ATPase in intracellular Ca2+ storage sites.

Spirotryprostatin A is an indolic alkaloid from the 2,5-Diketopiperazine class of natural products found in the Aspergillus fumigatus fungus. Spirotryprostatin A and several other indolic alkaloids have been found to have anti-mitotic properties, and as such they have become of great interest as anti-cancer drugs. Because of this, the total syntheses of these compounds is a major pursuit of organic chemists, and a number of different syntheses have been published in the chemical literature.
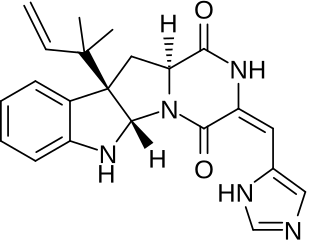
Roquefortine C is a mycotoxin that belongs to a class of naturally occurring 2,5-diketopiperazines produced by various fungi, particularly species from the genus Penicillium. It was first isolated from a strain of Penicillium roqueforti, a species commercially used as a source of proteolytic and lipolytic enzymes during maturation of the blue-veined cheeses, Roquefort, Danish Blue, Stilton and Gorgonzola.

Brevianamides are indole alkaloids that belong to a class of naturally occurring 2,5-diketopiperazines produced as secondary metabolites of fungi in the genus Penicillium and Aspergillus. Structurally similar to paraherquamides, they are a small class compounds that contain a bicyclo[2.2.2]diazoctane ring system. One of the major secondary metabolites in Penicillium spores, they are responsible for inflammatory response in lung cells.

(−)-Aurantiamine is a blue fluorescence metabolite produced by the fungus Penicillium aurantiogriseum, the most common fungi found in cereals. (−)-Aurantiamine belongs to a class of naturally occurring 2,5-diketopiperazines featuring a dehydrohistidine residue that exhibit important biological activities, such as anti-cancer or neurotoxic effects. It is the isopropyl analog of the microtubule binding agent (−)-phenylahistin but is 40 times less active than the latter on P388 cell proliferation. The total asymmetric synthesis of (−)-aurantiamine has been described.

Aurantiomides are quinazoline alkaloids isolated from the fungus Penicillium aurantiogriseum.

Fumitremorgins are tremorogenic metabolites of Aspergillus and Penicillium, that belong to a class of naturally occurring 2,5-diketopiperazines.
Fellutanine A, B, C and D are bio-active diketopiperazine alkaloids isolated from the cultures of Penicillium fellutanum, that belongs to a class of naturally occurring 2,5-diketopiperazines. Originally they were thought to be based on the "trans" cyclic dipetide cyclo(L-Trp-D-Trp) but were later shown to be based on the "cis" cyclic dipetide cyclo(L-Trp-L-Trp). This was also confirmed when fellutanine A, B and C were isolated from Penicillium simplicissimum. The fellutanines A−C, are non-annulated analogues of cyclo(L-Trp-L-Trp), but unlike their diannulated analogue fellutanine D are not cytotoxic.

Citromycin is a chemical compound produced by Penicillium. It was first discovered in 1969 and was found to have weak antibiotic activity.

Verruculogen is a mycotoxin produced by certain strains of aspergillus that belongs to a class of naturally occurring 2,5-diketopiperazines. It is an annulated analogue of cyclo(L-Trp-L-Pro) which belongs to the most abundant and structurally diverse class of tryptophan-proline 2,5-diketopiperazine natural products. It produces tremors in mice due to its neurotoxic properties. It also tested positive in a Salmonella/mammalian microsome assay and was shown to be genotoxic. It is a potent blocker of calcium-activated potassium channels.

Citromycetin is a bio-active polyketide isolated from Australian Penicillium.
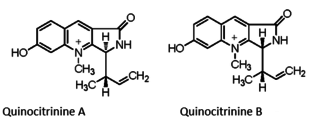
Quinocitrinines are quinoline alkaloids isolated from a permafrost Penicillium.
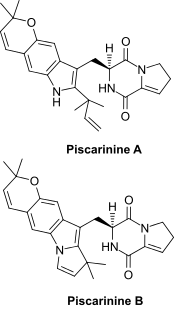
Piscarinines are bioactive alkaloid isolates of Penicillium piscarium NKM F-961 and Penicillium piscarium Westling that belong to a class of naturally occurring 2,5-diketopiperazines. The cytotoxic dehydroproline tryptophan derivatives piscarinines A and B were shown to be active against the prostate cancer cell line LNCAP.

Stephacidin A and B are antitumor alkaloids isolated from the fungus Aspergillus ochraceus that belong to a class of naturally occurring 2,5-diketopiperazines. This unusual family of fungal metabolites are complex bridged 2,5-diketopiperazine alkaloids that possess a unique bicyclo[2.2.2]diazaoctane core ring system and are constituted mainly from tryptophan, proline, and substituted proline derivatives where the olefinic unit of the isoprene moiety has been formally oxidatively cyclized across the α-carbon atoms of a 2,5-diketopiperazine ring. The molecular architecture of stephacidin B, formally a dimer of avrainvillamide, reveals a complex dimeric prenylated N-hydroxyindole alkaloid that contains 15 rings and 9 stereogenic centers and is one of the most complex indole alkaloids isolated from fungi. Stephacidin B rapidly converts into the electrophilic monomer avrainvillamide in cell culture, and there is evidence that the monomer avrainvillamide interacts with intracellular thiol-containing proteins, most likely by covalent modification.

(-)-Versicolamide B and (+)-Versicolamide B are spiroindole alkaloids isolated from the fungus Aspergillus that belong to a class of naturally occurring 2,5-diketopiperazines. The versicolamides are structurally complex spiro-cyclized versions of prenylated cyclo(L-Trp-L-Pro) derivatives which possess a unique spiro-fusion to a pyrrolidine at the 3-position of the oxindole core together with the bicyclo[2.2.2]diazaoctane ring system. While (-)-versicolamide B was isolated from the marine fungus Aspergillus sp. the enantiomer (+)-versicolamide B was isolated from the terrestrial fungi Aspergillus versicolor NRRL. The total asymmetric syntheses of both enantiomers have been achieved and the implications of their biosynthesis have been investigated.

Dideoxyverticillin A (+)-11,11’-dideoxyverticillin A is a complex epipolythiodioxopiperazine initially isolated from the marine fungus Penicillium sp. in 1999 has also been found in the marine fungus (Bionectriaceae), and belongs to a class of naturally occurring 2,5-diketopiperazines. Dideoxyverticillin A potently inhibits the tyrosine kinase activity of the epidermal growth factor receptor, exhibits antiangiogenic activity, and has efficacy against several cancer cell lines. Its reported anticancer mechanism is that it acts as a farnesyl transferase inhibitor. Dozens of semi-synthetic anticancer compounds have been made from Dideoxyverticillin A. Dimeric derivatives are reported to have better anticancer activity. The enantioselective first total synthesis of (+)-11,11’-dideoxyverticillin A, the structure of which contains many sterically congested, contiguous stereogenic centers as well as acid- and base-labile and redox-sensitive functionality, was biosynthetically inspired and achieved with high levels of chemical sophistication.
Penicillium dipodomyis is a species of the genus of Penicillium which occurs in kangaroo rats and produces penicillin and the diketopiperazine dipodazine.
Penicillium rugulosum is an anamorph species of fungus in the genus Penicillium which produces inulinase, luteoskyrin and (+) rugulosin.
Penicillium striatisporum is an anamorph species of fungus in the genus Penicillium which was isolated from the rhizosphere of chilli peppers. Penicillium striatisporum has an selective antifungal activity against Candida albicans This species produces striatisporin A, striatisporolide A, versiol, calbistrin C, deformylcalbistrin A, citromycetin, citromycin, fulvic acid, (-)-2,3-dihydrocitromycetin and (+)-hexylitaconic acid
Penicillium vinaceum is an anamorph species of fungus in the genus Penicillium which produces penicillivinacine, vinaxanthone and citrmycetin.
















