Related Research Articles

Imatinib, sold under the brand names Gleevec and Glivec (both marketed worldwide by Novartis) among others, is an oral targeted therapy medication used to treat cancer. Imatinib is a small molecule inhibitor targeting multiple tyrosine kinases such as CSF1R, ABL, c-KIT, FLT3, and PDGFR-β. Specifically, it is used for chronic myelogenous leukemia (CML) and acute lymphocytic leukemia (ALL) that are Philadelphia chromosome–positive (Ph+), certain types of gastrointestinal stromal tumors (GIST), hypereosinophilic syndrome (HES), chronic eosinophilic leukemia (CEL), systemic mastocytosis, and myelodysplastic syndrome.

In the fields of medicine, biotechnology and pharmacology, drug discovery is the process by which new candidate medications are discovered.

Bromocriptine, originally marketed as Parlodel and subsequently under many brand names, is an ergoline derivative and dopamine agonist that is used in the treatment of pituitary tumors, Parkinson's disease, hyperprolactinaemia, neuroleptic malignant syndrome, and, as an adjunct, type 2 diabetes.

Enalapril, sold under the brand name Vasotec among others, is an ACE inhibitor medication used to treat high blood pressure, diabetic kidney disease, and heart failure. For heart failure, it is generally used with a diuretic, such as furosemide. It is given by mouth or by injection into a vein. Onset of effects are typically within an hour when taken by mouth and last for up to a day.

Thiazide refers to both a class of sulfur-containing organic molecules and a class of diuretics based on the chemical structure of benzothiadiazine. The thiazide drug class was discovered and developed at Merck and Co. in the 1950s. The first approved drug of this class, chlorothiazide, was marketed under the trade name Diuril beginning in 1958. In most countries, thiazides are the least expensive antihypertensive drugs available.

Fingolimod, sold under the brand name Gilenya, is an immunomodulating medication, mostly used for treating multiple sclerosis (MS). Fingolimod is a sphingosine-1-phosphate receptor modulator, which sequesters lymphocytes in lymph nodes, preventing them from contributing to an autoimmune reaction. It has been reported to reduce the rate of relapses in relapsing-remitting multiple sclerosis by approximately one-half over a two-year period.
Chelerythrine is a benzophenanthridine alkaloid present in the plant Chelidonium majus. It is a potent, selective, and cell-permeable protein kinase C inhibitor in vitro. And an efficacious antagonist of G-protein-coupled CB1 receptors. This molecule also exhibits anticancer qualities and it has served as a base for many potential novel drugs against cancer. Structurally, this molecule has two distinct conformations, one being a positively charged iminium form, and the other being an uncharged form, a pseudo-base.

Rasagiline is an irreversible inhibitor of monoamine oxidase-B used as a monotherapy to treat symptoms in early Parkinson's disease or as an adjunct therapy in more advanced cases.
p38 mitogen-activated protein kinases are a class of mitogen-activated protein kinases (MAPKs) that are responsive to stress stimuli, such as cytokines, ultraviolet irradiation, heat shock, and osmotic shock, and are involved in cell differentiation, apoptosis and autophagy. Persistent activation of the p38 MAPK pathway in muscle satellite cells due to ageing, impairs muscle regeneration.
The ErbB family of proteins contains four receptor tyrosine kinases, structurally related to the epidermal growth factor receptor (EGFR), its first discovered member. In humans, the family includes Her1, Her2 (ErbB2), Her3 (ErbB3), and Her4 (ErbB4). The gene symbol, ErbB, is derived from the name of a viral oncogene to which these receptors are homologous: erythroblastic leukemia viral oncogene. Insufficient ErbB signaling in humans is associated with the development of neurodegenerative diseases, such as multiple sclerosis and Alzheimer's disease, while excessive ErbB signaling is associated with the development of a wide variety of types of solid tumor.

Glucose-6-phosphate exchanger SLC37A4, also known as glucose-6-phosphate translocase, is an enzyme that in humans is encoded by the SLC37A4 gene.
Glucagon-like peptide-1 receptor agonists, also known as GLP-1 receptor agonists(GLP-1-RA), incretin mimetics, or GLP-1 analogs, are agonists of the GLP-1 receptor. This class of medications is used for the treatment of type 2 diabetes. One of their advantages over older insulin secretagogues, such as sulfonylureas or meglitinides, is that they have a lower risk of causing hypoglycemia. GLP-1 has a short duration of action, so to overcome this limitation several modifications in either the drugs or the formulations are being developed. The 2022 ADA standards of medical care in diabetes include GLP-1-RA as a first line pharmacological therapy for type 2 diabetes, specifically in patients with atherosclerotic cardiovascular disease or obesity.

Lipidology is the scientific study of lipids. Lipids are a group of biological macromolecules that have a multitude of functions in the body. Clinical studies on lipid metabolism in the body have led to developments in therapeutic lipidology for disorders such as cardiovascular disease.

The 3C-like protease (3CLpro) or main protease (Mpro), formally known as C30 endopeptidase or 3-chymotrypsin-like protease, is the main protease found in coronaviruses. It cleaves the coronavirus polyprotein at eleven conserved sites. It is a cysteine protease and a member of the PA clan of proteases. It has a cysteine-histidine catalytic dyad at its active site and cleaves a Gln–(Ser/Ala/Gly) peptide bond.

A deuterated drug is a small molecule medicinal product in which one or more of the hydrogen atoms contained in the drug molecule have been replaced by its heavier stable isotope deuterium. Because of the kinetic isotope effect, deuterium-containing drugs may have significantly lower rates of metabolism, and hence a longer half-life.
SGLT2 inhibitors, also called gliflozins or flozins, are a class of medications that modulate sodium-glucose transport proteins in the nephron, unlike SGLT1 inhibitors that perform a similar function in the intestinal mucosa. The foremost metabolic effect of this is to inhibit reabsorption of glucose in the kidney and therefore lower blood sugar. They act by inhibiting sodium-glucose transport protein 2 (SGLT2). SGLT2 inhibitors are used in the treatment of type II diabetes mellitus (T2DM). Apart from blood sugar control, gliflozins have been shown to provide significant cardiovascular benefit in patients with type II diabetes (T2DM). Several medications of this class have been approved or are currently under development. In studies on canagliflozin, a member of this class, the medication was found to enhance blood sugar control as well as reduce body weight and systolic and diastolic blood pressure.
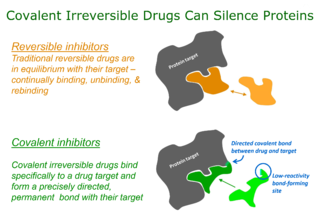
Targeted covalent inhibitors (TCIs) or Targeted covalent drugs are rationally designed inhibitors that bind and then bond to their target proteins. These inhibitors possess a bond-forming functional group of low chemical reactivity that, following binding to the target protein, is positioned to react rapidly with a proximate nucleophilic residue at the target site to form a bond.
A proteolysis targeting chimera (PROTAC) is a heterobifunctional molecule composed of two active domains and a linker, capable of removing specific unwanted proteins. Rather than acting as a conventional enzyme inhibitor, a PROTAC works by inducing selective intracellular proteolysis. PROTACs consist of two covalently linked protein-binding molecules: one capable of engaging an E3 ubiquitin ligase, and another that binds to a target protein meant for degradation. Recruitment of the E3 ligase to the target protein results in ubiquitination and subsequent degradation of the target protein via the proteasome. Because PROTACs need only to bind their targets with high selectivity, there are currently many efforts to retool previously ineffective inhibitor molecules as PROTACs for next-generation drugs.

Halicin (SU-3327) is an experimental drug that acts as an enzyme inhibitor of c-Jun N-terminal kinase (JNK). Originally, it was researched for the treatment of diabetes, but development was discontinued for this application due to poor results in testing. In 2019, this molecule was found by an artificial intelligence (AI) model to show antibiotic properties against a number of bacteria.

Hagit Eldar-Finkelman is an Israeli scientist and a principal investigator of an active research laboratory at the Sackler School of Medicine at Tel Aviv University. Eldar-Finkelman’s research is focused on the signal transduction field and drug development targeting protein kinases. She is well known for her pioneering work on the functions of GSK-3 and its contribution to diabetes and other pathogenies, including depressive behavior, Alzheimer’s diseases, and Huntington’s diseases. Novel findings also include the unique evolution of GSK-3 isozymes. Eldar-Finkelman is a leading figure in developing novel substrate competitive inhibitors (SCIs) for GSK-3 with significant benefits as drug candidates.
References
- ↑ "Russell Dahl". Santa Clara Magazine. Retrieved 2023-08-22.
- ↑ "Chemistry Tree - Russell S. Dahl". academictree.org. Retrieved 2023-08-22.
- ↑ "Russel Dahl - Biography".
- ↑ "Learn More About Dr. Russel Dahl / His Role In Calcium ATPase Research". Brunde Broady. Retrieved 2023-08-22.
- ↑ "Insights of Pharmacology and Pharmaceutical Science Editor Details". scholars.direct. Retrieved 2023-08-22.
- ↑ Dahl, Russell S.; Finney, Nathaniel S. (2004-07-14). "A surprising dipolar cycloaddition provides ready access to aminoglycosides". Journal of the American Chemical Society. 126 (27): 8356–8357. doi:10.1021/ja0319238. ISSN 0002-7863. PMID 15237974.
- ↑ Dahl, Russell; Baldridge, Kim K.; Finney, Nathaniel S. (July 2010). "Efficient Access to Aminomannoside Derivatives via Formal [2+2] Cycloaddition of Triazolinediones and Tri-O-acetyl-d-glucal". Synthesis. 2010 (13): 2292–2296. doi:10.1055/s-0029-1218803. ISSN 0039-7881.
- ↑ Dahl, Russell S.; Finney, Nathaniel S. (2001-07-01). "Simple Glucal-Based Linker for the Immobilization of Alcohols on Solid Support". Journal of Combinatorial Chemistry. 3 (4): 329–331. doi:10.1021/cc0100058. ISSN 1520-4766. PMID 11442388.
- ↑ "Russell Dahl". Santa Clara Magazine. Retrieved 2023-08-22.
- ↑ "Science Prerequisites Faculty & Staff". UNE Online. Retrieved 2023-08-22.
- ↑ "Google Patents". patents.google.com. Retrieved 2023-08-22.
- ↑ Hovnanian, A. (2007), Carafoli, Ernesto; Brini, Marisa (eds.), "Serca pumps and human diseases", Calcium Signalling and Disease, Subcellular Biochemistry, Dordrecht: Springer Netherlands, vol. 45, pp. 337–363, doi:10.1007/978-1-4020-6191-2_12, ISBN 978-1-4020-6190-5, PMID 18193643 , retrieved 2023-08-22
- ↑ "Russell Dahl Inventions, Patents and Patent Applications - Justia Patents Search". patents.justia.com. Retrieved 2023-08-22.
- ↑ "Neurodon | Publications". Neurodon. Retrieved 2023-08-22.
- ↑ Cornea, Razvan L.; Gruber, Simon J.; Lockamy, Elizabeth L.; Muretta, Joseph M.; Jin, Dongzhu; Chen, Jiqiu; Dahl, Russell; Bartfai, Tamas; Zsebo, Krisztina M.; Gillispie, Gregory D.; Thomas, David D. (January 2013). "High-throughput FRET assay yields allosteric SERCA activators". Journal of Biomolecular Screening. 18 (1): 97–107. doi:10.1177/1087057112456878. ISSN 1552-454X. PMC 3721969 . PMID 22923787.
- ↑ Gruber, Simon J.; Cornea, Razvan L.; Li, Ji; Peterson, Kurt C.; Schaaf, Tory M.; Gillispie, Gregory D.; Dahl, Russell; Zsebo, Krisztina M.; Robia, Seth L.; Thomas, David D. (February 2014). "Discovery of enzyme modulators via high-throughput time-resolved FRET in living cells". Journal of Biomolecular Screening. 19 (2): 215–222. doi:10.1177/1087057113510740. ISSN 1552-454X. PMC 4013825 . PMID 24436077.
- ↑ "Russell Dahl". scholar.google.com. Retrieved 2023-08-22.
- ↑ "Russell Dahl - CEO & Founder at Neurodon". THE ORG. Retrieved 2023-08-22.
- ↑ Montalban, Antonio Garrido; Boman, Erik; Chang, Chau-Dung; Ceide, Susana Conde; Dahl, Russell; Dalesandro, David; Delaet, Nancy G. J.; Erb, Eric; Ernst, Justin T.; Gibbs, Andrew; Kahl, Jeffrey (2010-08-15). "Optimization of alpha-ketoamide based p38 inhibitors through modifications to the region that binds to the allosteric site". Bioorganic & Medicinal Chemistry Letters. 20 (16): 4819–4824. doi:10.1016/j.bmcl.2010.06.102. ISSN 1464-3405. PMID 20663667.
- ↑ Montalban, Antonio Garrido; Boman, Erik; Chang, Chau-Dung; Ceide, Susana Conde; Dahl, Russell; Dalesandro, David; Delaet, Nancy G. J.; Erb, Eric; Ernst, Justin; Gibbs, Andrew; Kahl, Jeffrey (2010-04-25). "KR-003048, a potent, orally active inhibitor of p38 mitogen-activated protein kinase". European Journal of Pharmacology. 632 (1–3): 93–102. doi:10.1016/j.ejphar.2010.01.011. ISSN 1879-0712. PMID 20132813.
- ↑ Montalban, Antonio Garrido; Boman, Erik; Chang, Chau-Dung; Ceide, Susana Conde; Dahl, Russell; Dalesandro, David; Delaet, Nancy G. J.; Erb, Eric; Gibbs, Andrew; Kahl, Jeff; Kessler, Linda (2008-10-15). "'Reverse' alpha-ketoamide-based p38 MAP kinase inhibitors". Bioorganic & Medicinal Chemistry Letters. 18 (20): 5456–5459. doi:10.1016/j.bmcl.2008.09.028. ISSN 1464-3405. PMID 18835164.
- ↑ Montalban, Antonio Garrido; Boman, Erik; Chang, Chau-Dung; Ceide, Susana Conde; Dahl, Russell; Dalesandro, David; Delaet, Nancy G. J.; Erb, Eric; Ernst, Justin T.; Gibbs, Andrew; Kahl, Jeffrey (2008-03-15). "The design and synthesis of novel alpha-ketoamide-based p38 MAP kinase inhibitors". Bioorganic & Medicinal Chemistry Letters. 18 (6): 1772–1777. doi:10.1016/j.bmcl.2008.02.033. ISSN 1464-3405. PMID 18325768.
- ↑ "Molecule could improve memory, reduce Alzheimer's degradation, study finds". ScienceDaily. Retrieved 2023-08-22.
- ↑ Dahl, Russell; Moore, Amanda C.; Knight, Caitlynn; Mauger, Colleen; Zhang, Hua; Schiltz, Gary E.; Koss, Wendy A.; Bezprozvanny, Ilya (January 2023). "Positive Allosteric Modulator of SERCA Pump NDC-1173 Exerts Beneficial Effects in Mouse Model of Alzheimer's Disease". International Journal of Molecular Sciences. 24 (13): 11057. doi: 10.3390/ijms241311057 . ISSN 1422-0067. PMC 10341805 . PMID 37446234.
- ↑ Whooley, Sean (2021-05-17). "Neurodon prepping IND application for type 1 diabetes treatment". Drug Delivery Business. Retrieved 2023-08-22.
- ↑ Foundation, Purdue Research (2022-07-07). "Take 6: Russell Dahl, Neurodon". The Line by PRF. Retrieved 2023-08-22.
- ↑ Veleta, Kylie. "Startup awarded nearly $1M to advance diabetes drug". Inside INdiana Business. Retrieved 2023-08-22.
- ↑ "Neurodon Announces Initiation of Investigational New Drug (IND)-Enabling Studies for NDC-0009, a Novel Small Molecule for the Treatment of Type 1 Diabetes (T1D)". www.businesswire.com. 2021-05-17. Retrieved 2023-08-22.
- ↑ "Drug Discovery and Molecular Pharmacology B Study Section ROSTER". public.era.nih.gov. Retrieved 2023-08-22.
- ↑ "NIA Small Business Showcase: Neurodon, LLC". National Institute on Aging. Retrieved 2023-08-22.