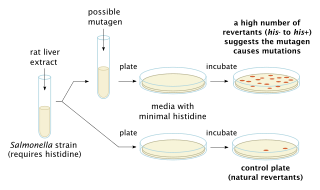
The Ames test is a widely employed method that uses bacteria to test whether a given chemical can cause mutations in the DNA of the test organism. More formally, it is a biological assay to assess the mutagenic potential of chemical compounds. A positive test indicates that the chemical is mutagenic and therefore may act as a carcinogen, because cancer is often linked to mutation. The test serves as a quick and convenient assay to estimate the carcinogenic potential of a compound because standard carcinogen assays on mice and rats are time-consuming and expensive. However, false-positives and false-negatives are known.

In vitro studies are performed with microorganisms, cells, or biological molecules outside their normal biological context. Colloquially called "test-tube experiments", these studies in biology and its subdisciplines are traditionally done in labware such as test tubes, flasks, Petri dishes, and microtiter plates. Studies conducted using components of an organism that have been isolated from their usual biological surroundings permit a more detailed or more convenient analysis than can be done with whole organisms; however, results obtained from in vitro experiments may not fully or accurately predict the effects on a whole organism. In contrast to in vitro experiments, in vivo studies are those conducted in living organisms, including humans, and whole plants.

In genetics, a mutagen is a physical or chemical agent that permanently changes genetic material, usually DNA, in an organism and thus increases the frequency of mutations above the natural background level. As many mutations can cause cancer, such mutagens are therefore carcinogens, although not all necessarily are. All mutagens have characteristic mutational signatures with some chemicals becoming mutagenic through cellular processes. Not all mutations are caused by mutagens: so-called "spontaneous mutations" occur due to spontaneous hydrolysis, errors in DNA replication, repair and recombination.

Pharmacology is a branch of medicine and pharmaceutical sciences concerned with drug or medication action, where a drug may be defined as any artificial, natural, or endogenous molecule which exerts a biochemical or physiological effect on the cell, tissue, organ, or organism. More specifically, it is the study of the interactions that occur between a living organism and chemicals that affect normal or abnormal biochemical function. If substances have medicinal properties, they are considered pharmaceuticals.

Ethidium bromide is an intercalating agent commonly used as a fluorescent tag in molecular biology laboratories for techniques such as agarose gel electrophoresis. It is commonly abbreviated as EtBr, which is also an abbreviation for bromoethane. To avoid confusion, some laboratories have used the abbreviation EthBr for this salt. When exposed to ultraviolet light, it will fluoresce with an orange colour, intensifying almost 20-fold after binding to DNA. Under the name homidium, it has been commonly used since the 1950s in veterinary medicine to treat trypanosomiasis in cattle. The high incidence of antimicrobial resistance makes this treatment impractical in some areas, where the related isometamidium chloride is used instead. Ethidium bromide may be a mutagen, although this depends on the organism exposed and the circumstances of exposure.
In genetics, genotoxicity describes the property of chemical agents that damages the genetic information within a cell causing mutations, which may lead to cancer. While genotoxicity is often confused with mutagenicity, all mutagens are genotoxic, whereas not all genotoxic substances are mutagenic. The alteration can have direct or indirect effects on the DNA: the induction of mutations, mistimed event activation, and direct DNA damage leading to mutations. The permanent, heritable changes can affect either somatic cells of the organism or germ cells to be passed on to future generations. Cells prevent expression of the genotoxic mutation by either DNA repair or apoptosis; however, the damage may not always be fixed leading to mutagenesis.

Earl Wilbur Sutherland Jr. was an American pharmacologist and biochemist born in Burlingame, Kansas. Sutherland won a Nobel Prize in Physiology or Medicine in 1971 "for his discoveries concerning the mechanisms of the action of hormones", especially epinephrine, via second messengers, namely cyclic adenosine monophosphate, or cyclic AMP.
In cell biology, microsomes are heterogenous vesicle-like artifacts re-formed from pieces of the endoplasmic reticulum (ER) when eukaryotic cells are broken-up in the laboratory; microsomes are not present in healthy, living cells.

Methylcholanthrene is a highly carcinogenic polycyclic aromatic hydrocarbon produced by burning organic compounds at very high temperatures. Methylcholanthrene is also known as 3-methylcholanthrene, 20-methylcholanthrene or the IUPAC name 3-methyl-1,2-dyhydrobenzo[j]aceanthrylene. The short notation often used is 3-MC or MCA. This compound forms pale yellow solid crystals when crystallized from benzene and ether. It has a melting point around 180 °C and its boiling point is around 280 °C at a pressure of 80 mmHg. Methylcholanthrene is used in laboratory studies of chemical carcinogenesis. It is an alkylated derivative of benz[a]anthracene and has a similar UV spectrum. The most common isomer is 3-methylcholanthrene, although the methyl group can occur in other places.
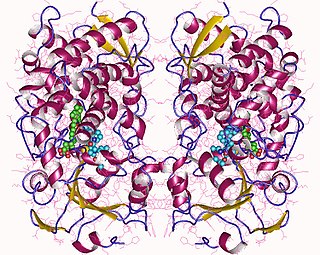
Cytochrome P450 3A4 is an important enzyme in the body, mainly found in the liver and in the intestine. It oxidizes small foreign organic molecules (xenobiotics), such as toxins or drugs, so that they can be removed from the body.
Drug metabolism is the metabolic breakdown of drugs by living organisms, usually through specialized enzymatic systems. More generally, xenobiotic metabolism is the set of metabolic pathways that modify the chemical structure of xenobiotics, which are compounds foreign to an organism's normal biochemistry, such as any drug or poison. These pathways are a form of biotransformation present in all major groups of organisms and are considered to be of ancient origin. These reactions often act to detoxify poisonous compounds. The study of drug metabolism is called pharmacokinetics.

Sudan I, is an organic compound, typically classified as an azo dye. It is an intensely orange-red solid that is added to colourise waxes, oils, petrol, solvents, and polishes. Sudan I has also been adopted for colouring various foodstuffs, especially curry powder and chili powder, although the use of Sudan I in foods is now banned in many countries, because Sudan I, Sudan III, and Sudan IV have been classified as category 3 carcinogens by the International Agency for Research on Cancer. Sudan I is still used in some orange-coloured smoke formulations and as a colouring for cotton refuse used in chemistry experiments.
Toxicogenomics is a subdiscipline of pharmacology that deals with the collection, interpretation, and storage of information about gene and protein activity within a particular cell or tissue of an organism in response to exposure to toxic substances. Toxicogenomics combines toxicology with genomics or other high-throughput molecular profiling technologies such as transcriptomics, proteomics and metabolomics. Toxicogenomics endeavors to elucidate the molecular mechanisms evolved in the expression of toxicity, and to derive molecular expression patterns that predict toxicity or the genetic susceptibility to it.

Cytochrome P450 family 2 subfamily C member 9 is an enzyme protein. The enzyme is involved in metabolism, by oxidation, of both xenobiotics, including drugs, and endogenous compounds, including fatty acids. In humans, the protein is encoded by the CYP2C9 gene. The gene is highly polymorphic, which affects the efficiency of the metabolism by the enzyme.
Integrated discrete Multiple Organ Culture (IdMOC) is an in vitro, cell culture based experimental model for the study of intercellular communication. In conventional in vitro systems, each cell type is studied in isolation ignoring critical interactions between organs or cell types. IdMOC technology is based on the concept that multiple organs signal or communicate via the systemic circulation.
Cytochrome P450, family 3, subfamily A, also known as CYP3A, is a human gene locus. A homologous locus is found in mice.
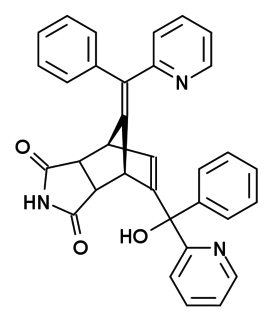
Norbormide is a toxic compound used as a rodenticide. It has several mechanisms of action, acting as a vasoconstrictor and calcium channel blocker, but is selectively toxic to rats and has relatively low toxicity to other species, due to a species specific action of opening the permeability transition pores in rat mitochondria.
Albert P. Li is president and CEO of In Vitro ADMET Laboratories (IVAL), Columbia, Maryland, and Malden, Massachusetts. For the past three decades, Li has devoted his scientific career to the advancement of scientific concepts and technologies to accurately predict human drug properties. His research is focused on the development and application of human-based in vitro experimental systems in drug discovery and development. He is a pioneer in the isolation, cryopreservation, and culturing of human hepatocytes and their application in the evaluation of drug metabolism, drug-drug interactions, and drug toxicity.
Bisphenol F is a small aromatic organic compound with the chemical formula (HOC
6H
4)
2CH
2. It is related to bisphenol A through its basic structure, as both belong to the category of molecules known as bisphenols, which feature two phenol groups connected via a linking group. In BPF, the two aromatic rings are linked by a methylene connecting group.

EG-018 is a carbazole-based synthetic cannabinoid that has been sold online as a designer drug. It acts as a partial agonist of the CB1 and CB2 receptor, with reasonably high binding affinity, but low efficacy in terms of inducing a signaling response.











