Radiology is the medical discipline that uses medical imaging to diagnose and treat diseases within the bodies of animals and humans.

Nuclear medicine is a medical specialty involving the application of radioactive substances in the diagnosis and treatment of disease. Nuclear medicine imaging, in a sense, is "radiology done inside out" or "endoradiology" because it records radiation emitting from within the body rather than radiation that is generated by external sources like X-rays. In addition, nuclear medicine scans differ from radiology, as the emphasis is not on imaging anatomy, but on the function. For such reason, it is called a physiological imaging modality. Single photon emission computed tomography (SPECT) and positron emission tomography (PET) scans are the two most common imaging modalities in nuclear medicine.
Amyloidosis is a group of diseases in which abnormal proteins, known as amyloid fibrils, build up in tissue. There are several non-specific and vague signs and symptoms associated with amyloidosis. These include: fatigue, peripheral edema, weight loss, shortness of breath, palpitations, and feeling faint with standing. In AL amyloidosis, specific symptoms can include enlargement of the tongue and periorbital purpura. In wild-type ATTR amyloidosis, non-cardiac symptoms include: bilateral carpal tunnel syndrome, lumbar spinal stenosis, bicep tendon rupture, small fiber neuropathy, and autonomic dysfunction.

Scintigraphy, also known as a gamma scan, is a diagnostic test in nuclear medicine, where radioisotopes attached to drugs that travel to a specific organ or tissue (radiopharmaceuticals) are taken internally and the emitted gamma radiation is captured by external detectors to form two-dimensional images in a similar process to the capture of x-ray images. In contrast, SPECT and positron emission tomography (PET) form 3-dimensional images and are therefore classified as separate techniques from scintigraphy, although they also use gamma cameras to detect internal radiation. Scintigraphy is unlike a diagnostic X-ray where external radiation is passed through the body to form an image.

Bence Jones protein is a monoclonal globulin protein or immunoglobulin light chain found in the urine, with a molecular weight of 22–24 kDa. Detection of Bence Jones protein may be suggestive of multiple myeloma or Waldenström's macroglobulinemia.

Thyroid disease is a medical condition that affects the function of the thyroid gland. The thyroid gland is located at the front of the neck and produces thyroid hormones that travel through the blood to help regulate many other organs, meaning that it is an endocrine organ. These hormones normally act in the body to regulate energy use, infant development, and childhood development.

The serum amyloid P component (SAP) is the identical serum form of amyloid P component (AP), a 25kDa pentameric protein first identified as the pentagonal constituent of in vivo pathological deposits called "amyloid". APCS is its human gene.

Familial amyloid polyneuropathy, also called transthyretin-related hereditary amyloidosis, transthyretin amyloidosis abbreviated also as ATTR, or Corino de Andrade's disease, is an autosomal dominant neurodegenerative disease. It is a form of amyloidosis, and was first identified and described by Portuguese neurologist Mário Corino da Costa Andrade, in 1952. FAP is distinct from senile systemic amyloidosis (SSA), which is not inherited, and which was determined to be the primary cause of death for 70% of supercentenarians who have been autopsied. FAP can be ameliorated by liver transplantation.

Cardiac amyloidosis is a subcategory of amyloidosis where there is depositing of the protein amyloid in the cardiac muscle and surrounding tissues. Amyloid, a misfolded and insoluble protein, can become a deposit in the heart’s atria, valves, or ventricles. These deposits can cause thickening of different sections of the heart, leading to decreased cardiac function. The overall decrease in cardiac function leads to a plethora of symptoms. This multisystem disease was often misdiagnosed, with diagnosis previously occurring after death during autopsy. However, recent advancements of technologies have increased the diagnosis of the disease. Cardiac amyloidosis has multiple sub-types including light chain, familial, and senile. One of the most studied types is light chain cardiac amyloidosis. Prognosis depends on the extent of the deposits in the body and the type of amyloidosis. New treatment methods are actively being researched in regards to the treatment of heart failure and specific cardiac amyloidosis problems.

Ioflupane (123I) is the international nonproprietary name (INN) of a cocaine analogue which is a neuro-imaging radiopharmaceutical drug, used in nuclear medicine for the diagnosis of Parkinson's disease and the differential diagnosis of Parkinson's disease over other disorders presenting similar symptoms. During the DaT scan procedure it is injected into a patient and viewed with a gamma camera in order to acquire SPECT images of the brain with particular respect to the striatum, a subcortical region of the basal ganglia. The drug is sold under the brand name Datscan and is manufactured by GE Healthcare, formerly Amersham plc.

Leukocyte cell-derived chemotaxin-2 (LECT2) is a protein first described in 1996 as a chemotactic factor for neutrophils, i.e. it stimulated human neutrophils to move directionally in an in vitro assay system. The protein was detected in and purified from cultures of Phytohaemagglutinin-activated human T-cell leukemia SKW-3 cells. Subsequent studies have defined LECT2 as a hepatokine, i.e. a substance made and released into the circulation by liver hepatocyte cells that regulates the function of other cells: it is a hepatocyte-derived, hormone-like, signaling protein.
Plasma cell dyscrasias are a spectrum of progressively more severe monoclonal gammopathies in which a clone or multiple clones of pre-malignant or malignant plasma cells over-produce and secrete into the blood stream a myeloma protein, i.e. an abnormal monoclonal antibody or portion thereof. The exception to this rule is the disorder termed non-secretory multiple myeloma; this disorder is a form of plasma cell dyscrasia in which no myeloma protein is detected in serum or urine of individuals who have clear evidence of an increase in clonal bone marrow plasma cells and/or evidence of clonal plasma cell-mediated tissue injury. Here, a clone of plasma cells refers to group of plasma cells that are abnormal in that they have an identical genetic identity and therefore are descendants of a single genetically distinct ancestor cell.
Amyloid light-chain (AL) amyloidosis, also known as primary amyloidosis, is the most common form of systemic amyloidosis in the US. The disease is caused when a person's antibody-producing cells do not function properly and produce abnormal protein fibers made of components of antibodies called light chains. These light chains come together to form amyloid deposits which can cause serious damage to different organs. Abnormal light chains in urine are sometimes referred to as "Bence Jones protein".
AA amyloidosis is a form of amyloidosis, a disease characterized by the abnormal deposition of fibers of insoluble protein in the extracellular space of various tissues and organs. In AA amyloidosis, the deposited protein is serum amyloid A protein (SAA), an acute-phase protein which is normally soluble and whose plasma concentration is highest during inflammation.
Amyloidosis is the accumulation on misfolded protein fibers in the body. This is very common condition associated with many of the chronic illness. Haemodialysis-associated amyloidosis is a form of systemic amyloidosis associated with chronic kidney failure. Even if this is common in CKD patients with chronic regular dialysis, it can be also seen in patient with CKD but have never dialysed too.

Amyloid purpura is a condition marked by bleeding under the skin (purpura) in some individuals with amyloidosis. Its cause is unknown, but coagulation defects caused by amyloid are thought to contribute.
Sir Mark Brian Pepys is a South African-born British academic of medicine. He was until 2011 Professor of Medicine at University College London and Head of Medicine at the Hampstead Campus and the Royal Free Hospital.

LECT2 Amyloidosis (ALECT2) is a form of amyloidosis caused by the LECT2 protein. It was found to be the third most common cause of amyloidosis in a set of more than 4,000 individuals studied at the Mayo Clinic; the first and second most common forms the disorder were AL amyloidosis and AA amyloidosis, respectively. Amyloidosis is a disorder in which the abnormal deposition of a protein in organs and/or tissues gradually leads to organ failure and/or tissue injury.
Wild-type transthyretin amyloid (WTTA), also known as senile systemic amyloidosis (SSA) and abbreviated as ATTR, is a disease that typically affects the heart and tendons of elderly people. It is caused by accumulation of a wild-type protein called transthyretin. This is in contrast to a related condition called transthyretin-related hereditary amyloidosis where a genetically mutated transthyretin protein tends to deposit at a much earlier age than in WTTA, due to abnormal conformation and bioprocessing. It belongs to a group of diseases called amyloidosis, chronic progressive conditions linked to abnormal deposition of normal or abnormal proteins, because these proteins are misshapen and cannot be properly degraded and eliminated by the cell metabolism.
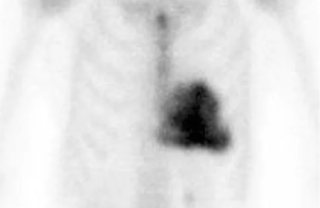
A DPD scan is a type of nuclear medicine imaging test which uses radioactive technetium-99m (99mTc) and 3,3-diphosphono-1,2-propanodicarboxylic acid (DPD) to diagnose cardiac amyloidosis. The radiopharmaceutical is taken up only in patients with ATTR amyloidosis, making it a useful tool to differentiate from AL amyloidosis.














