Related Research Articles

Cysteine (symbol Cys or C; ) is a semiessential proteinogenic amino acid with the formula HO2CCH(NH2)CH2SH. The thiol side chain in cysteine often participates in enzymatic reactions, as a nucleophile. The thiol is susceptible to oxidation to give the disulfide derivative cystine, which serves an important structural role in many proteins. When used as a food additive, it has the E number E920. It is encoded by the codons UGU and UGC.
In chemistry, a disulfide refers to a functional group with the structure R−S−S−R′. The linkage is also called an SS-bond or sometimes a disulfide bridge and is usually derived by the coupling of two thiol groups. In biology, disulfide bridges formed between thiol groups in two cysteine residues are an important component of the secondary and tertiary structure of proteins. The connection is a persulfide, in analogy to its congener, peroxide (R−O−O−R′), but this terminology is rarely used, except in reference to hydrodisulfides.

A thiol or thiol derivative is any organosulfur compound of the form R−SH, where R represents an alkyl or other organic substituent. The –SH functional group itself is referred to as either a thiol group or a sulfanyl group. Thiols are the sulfur analogue of alcohols, and the word is a portmanteau of "thio-" + "alcohol", with the first word deriving from Greek θεῖον (theion) meaning "sulfur".

An organic sulfide or thioether is a functional group in organosulfur chemistry with the connectivity C–S–C as shown on right. Like many other sulfur-containing compounds, volatile sulfides have foul odors. A sulfide is similar to an ether except that it contains a sulfur atom in place of the oxygen. The grouping of oxygen and sulfur in the periodic table suggests that the chemical properties of ethers and sulfides are somewhat similar, though the extent to which this is true in practice varies depending on the application.

In chemistry thioesters are compounds with the functional group R–S–CO–R'. They are analogous to carboxylate esters with the sulfur in the thioester playing the role of the linking oxygen in the carboxylate ester. They are the product of esterification between a carboxylic acid and a thiol. In biochemistry, the best-known thioesters are derivatives of coenzyme A, e.g., acetyl-CoA.
The prefix thio-, when applied to a chemical, such as an ion, means that an oxygen atom in the compound has been replaced by a sulfur atom. This term is often used in organic chemistry. For example, from the word ether, referring to an oxygen-containing compound having the general chemical structure R–O–R′, where R and R′ are organic functional groups and O is an oxygen atom, comes the word thioether, which refers to an analogous compound with the general structure R–S–R′, where S is a sulfur atom covalently bonded to two organic groups. A chemical reaction involving the replacement of oxygen to sulfur is called thionation or thiation.
Organosulfur compounds are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature abounds with organosulfur compounds—sulfur is essential for life. Of the 20 common amino acids, two are organosulfur compounds, and the antibiotics penicillin and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, the removal of which is a major focus of oil refineries.
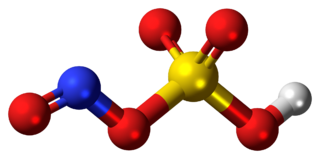
Nitrosylsulfuric acid is the chemical compound with the formula NOHSO4. It is a colourless solid that is used industrially in the production of caprolactam, and was formerly part of the lead chamber process for producing sulfuric acid. The compound is the mixed anhydride of sulfuric acid and nitrous acid.

Thiophenol is an organosulfur compound with the formula C6H5SH, sometimes abbreviated as PhSH. This foul-smelling colorless liquid is the simplest aromatic thiol. The chemical structures of thiophenol and its derivatives are analogous to phenols except the oxygen atom in the hydroxyl group (-OH) bonded to the aromatic ring is replaced by a sulfur atom. The prefix thio- implies a sulfur-containing compound and when used before a root word name for a compound which would normally contain an oxygen atom, in the case of 'thiol' that the alcohol oxygen atom is replaced by a sulfur atom.

Nitroso refers to a functional group in organic chemistry which has the NO group attached to an organic moiety. As such, various nitroso groups can be categorized as C-nitroso compounds (e.g., nitrosoalkanes; R−N=O), S-nitroso compounds (nitrosothiols; RS−N=O), N-nitroso compounds (e.g., nitrosamines, R1N(−R2)−N=O), and O-nitroso compounds (alkyl nitrites; RO−N=O).
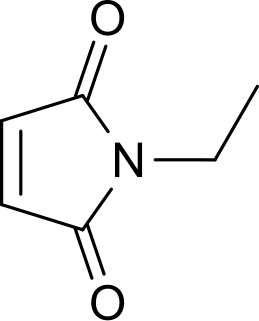
N-Ethylmaleimide (NEM) is an organic compound that is derived from maleic acid. It contains the amide functional group, but more importantly it is an alkene that is reactive toward thiols and is commonly used to modify cysteine residues in proteins and peptides.

Metal nitrosyl complexes are complexes that contain nitric oxide, NO, bonded to a transition metal. Many kinds of nitrosyl complexes are known, which vary both in structure and coligand.
Nitrosyl chloride is the chemical compound with the formula NOCl. It is a yellow gas that is most commonly encountered as a decomposition product of aqua regia, a mixture of hydrochloric acid and nitric acid. It is a strong electrophile and oxidizing agent. It is sometimes called Tilden's reagent.
The Barton reaction, also known as the Barton nitrite ester reaction, is a photochemical reaction that involves the photolysis of an alkyl nitrite to form a δ-nitroso alcohol.
In organic chemistry, the Baudisch reaction is a process for the synthesis of nitrosophenols using metal ions. Although the products are of limited value, the reaction is of historical interest as an example of metal-promoted functionalization of aromatic substrates.

Roussin’s Red Salt is the inorganic compound with the formula K2[Fe2S2(NO)4]. This metal nitrosyl was first described by Zacharie Roussin in 1858, making it one of the first synthetic iron-sulfur clusters.

Nitrosylation is the general term for covalent incorporation of a nitric oxide "nitrosyl" moiety into another molecule. There are multiple chemical mechanisms by which this can be achieved; including biological enzymes and industrial processes. The biological functions of nitrosylation are particularly important as S-nitrosylation, the conjugation of NO to cysteine thiols in proteins, is an important part of cell signalling. Coordination of NO to transition metals to give metal nitrosyl complexes, is also referred to as nitrosylation.
Nitrosyl bromide, is the chemical compound with the chemical formula NOBr. It is a red gas with a condensing point just below room temperature.

Sodium nitroprusside (SNP), sold under the brand name Nitropress among others, is a medication used to lower blood pressure. This may be done if the blood pressure is very high and resulting in symptoms, in certain types of heart failure, and during surgery to decrease bleeding. It is used by continuous injection into a vein. Onset is typically immediate and effects last for up to ten minutes.
The thiol-ene reaction is an organic reaction between a thiol and an alkene to form a thioether. This reaction was first reported in 1905, but it gained prominence in the late 1990s and early 2000s for its feasibility and wide range of applications. This reaction is accepted as a click chemistry reaction given the reactions’ high yield, stereoselectivity, high rate, and thermodynamic driving force.
References
- ↑ A scheme for the colorimetric determination of microgram amounts of thiols; Saville B. Analyst 83, 670 (1958) PN50818.