Filtration is a physical separation process that separates solid matter and fluid from a mixture using a filter medium that has a complex structure through which only the fluid can pass. Solid particles that cannot pass through the filter medium are described as oversize and the fluid that passes through is called the filtrate. Oversize particles may form a filter cake on top of the filter and may also block the filter lattice, preventing the fluid phase from crossing the filter, known as blinding. The size of the largest particles that can successfully pass through a filter is called the effective pore size of that filter. The separation of solid and fluid is imperfect; solids will be contaminated with some fluid and filtrate will contain fine particles. Filtration occurs both in nature and in engineered systems; there are biological, geological, and industrial forms. In everyday usage the verb "strain" is more often used; for example, using a colander to drain cooking water from cooked pasta.

Laboratory glassware is a variety of equipment used in scientific work, traditionally made of glass. Glass may be blown, bent, cut, molded, or formed into many sizes and shapes. It is commonly used in chemistry, biology, and analytical laboratories. Many laboratories have training programs to demonstrate how glassware is used and to alert first–time users to the safety hazards involved with using glassware.

A funnel is a tube or pipe that is wide at the top and narrow at the bottom, used for guiding liquid or powder into a small opening.

Sodium amide, commonly called sodamide, is the inorganic compound with the formula NaNH2. It is a salt composed of the sodium cation and the azanide anion. This solid, which is dangerously reactive toward water, is white, but commercial samples are typically gray due to the presence of small quantities of metallic iron from the manufacturing process. Such impurities do not usually affect the utility of the reagent. NaNH2 conducts electricity in the fused state, its conductance being similar to that of NaOH in a similar state. NaNH2 has been widely employed as a strong base in organic synthesis.

A gas syringe is a piece of laboratory glassware used to insert or withdraw a volume of a gas from a closed system, or to measure the volume of gas evolved from a chemical reaction. A gas syringe can also be used to measure and dispense liquids, especially where these liquids need to be kept free from air.

A Büchner funnel is a piece of laboratory equipment used in filtration. It is traditionally made of porcelain, but glass and plastic funnels are also available. On top of the funnel-shaped part there is a cylinder with a fritted glass disc/perforated plate separating it from the funnel. The Hirsch funnel has a similar design; it is used similarly, but for smaller quantities of material. The main difference is that the plate of a Hirsch funnel is much smaller, and the walls of the funnel angle outward instead of being vertical.

In electrochemistry, a salt bridge or ion bridge is an essential laboratory device discovered over 100 years ago. It contains an electrolyte solution, typically an inert solution, used to connect the oxidation and reduction half-cells of a galvanic cell, a type of electrochemical cell. In short, it functions as a link connecting the anode and cathode half-cells within an electrochemical cell. It also maintains electrical neutrality within the internal circuit and stabilizes the junction potential between the solutions in the half-cells. Additionally, it serves to minimize cross-contamination between the two half cells.

A stopcock is a form of valve used to control the flow of a liquid or gas. The term is not precise and is applied to many different types of valve. The only consistent attribute is that the valve is designed to completely stop the flow when closed fully.

The Schlenk line is a commonly used chemistry apparatus developed by Wilhelm Schlenk. It consists of a dual manifold with several ports. One manifold is connected to a source of purified inert gas, while the other is connected to a vacuum pump. The inert-gas line is vented through an oil bubbler, while solvent vapors and gaseous reaction products are prevented from contaminating the vacuum pump by a liquid-nitrogen or dry-ice/acetone cold trap. Special stopcocks or Teflon taps allow vacuum or inert gas to be selected without the need for placing the sample on a separate line.
A Schlenk flask, or Schlenk tube, is a reaction vessel typically used in air-sensitive chemistry, invented by Wilhelm Schlenk. It has a side arm fitted with a PTFE or ground glass stopcock, which allows the vessel to be evacuated or filled with gases. These flasks are often connected to Schlenk lines, which allow both operations to be done easily.

Agitated Nutsche filter (ANF) is a filtration technique used in applications such as dye, paint, and pharmaceutical production and waste water treatment. Safety requirements and environmental concerns due to solvent evaporation led to the development of this type of filter wherein filtration under vacuum or pressure can be carried out in closed vessels and solids can be discharged straightaway into a dryer.
Air-free techniques refer to a range of manipulations in the chemistry laboratory for the handling of compounds that are air-sensitive. These techniques prevent the compounds from reacting with components of air, usually water and oxygen; less commonly carbon dioxide and nitrogen. A common theme among these techniques is the use of a fine (100–10−3 Torr) or high (10−3–10−6 Torr) vacuum to remove air, and the use of an inert gas: preferably argon, but often nitrogen.
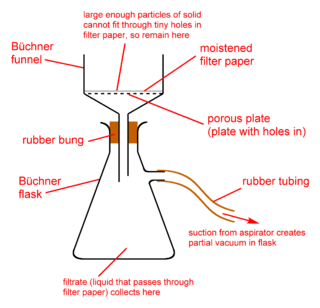
Vacuum filtration is a fast filtration technique used to separate solids from liquids.

Cannula transfer or cannulation is a set of air-free techniques used with a Schlenk line, in transferring liquid or solution samples between reaction vessels via cannulae, avoiding atmospheric contamination. While the syringes are not the same as cannulae, the techniques remain relevant.
A vacuum ceramic filter is designed to separate liquids from solids for dewatering of ore concentrates purposes. The device consists of a rotator, slurry tank, ceramic filter plate, distributor, discharge scraper, cleaning device, frame, agitating device, pipe system, vacuum system, automatic acid dosing system, automatic lubricating system, valve and discharge chute. The operation and construction principle of vacuum ceramic filter resemble those of a conventional disc filter, but the filter medium is replaced by a finely porous ceramic disc. The disc material is inert, has a long operational life and is resistant to almost all chemicals. Performance can be optimized by taking into account all those factors which affect the overall efficiency of the separation process. Some of the variables affecting the performance of a vacuum ceramic filter include the solid concentration, speed rotation of the disc, slurry level in the feed basin, temperature of the feed slurry, and the pressure during dewatering stages and filter cake formation.
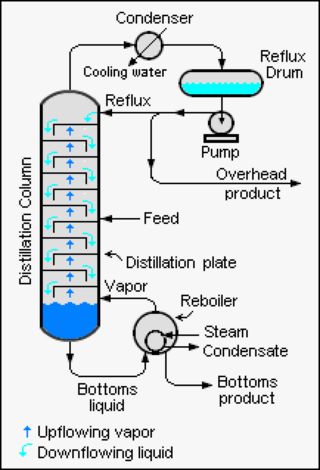
Reflux is a technique involving the condensation of vapors and the return of this condensate to the system from which it originated. It is used in industrial and laboratory distillations. It is also used in chemistry to supply energy to reactions over a long period of time.

Laboratory funnels are funnels that have been made for use in the chemical laboratory. There are many different kinds of funnels that have been adapted for these specialized applications. Filter funnels, thistle funnels, and dropping funnels have stopcocks which allow the fluids to be added to a flask slowly. For solids, a powder funnel with a short and wide neck/stem is more appropriate as it prevents clogging.
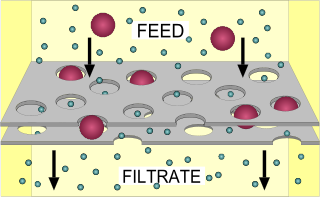
Membrane technology encompasses the scientific processes used in the construction and application of membranes. Membranes are used to facilitate the transport or rejection of substances between mediums, and the mechanical separation of gas and liquid streams. In the simplest case, filtration is achieved when the pores of the membrane are smaller than the diameter of the undesired substance, such as a harmful microorganism. Membrane technology is commonly used in industries such as water treatment, chemical and metal processing, pharmaceuticals, biotechnology, the food industry, as well as the removal of environmental pollutants.
A separation process is a method that converts a mixture or a solution of chemical substances into two or more distinct product mixtures, a scientific process of separating two or more substances in order to obtain purity. At least one product mixture from the separation is enriched in one or more of the source mixture's constituents. In some cases, a separation may fully divide the mixture into pure constituents. Separations exploit differences in chemical properties or physical properties between the constituents of a mixture.

Gravity filtration is a method of filtering impurities from solutions by using gravity to pull liquid through a filter. The two main kinds of filtration used in laboratories are gravity and vacuum/suction. Gravity filtration is often used in chemical laboratories to filter precipitates from precipitation reactions as well as drying agents, inadmissible side items, or remaining reactants. While it can also be used to separate out strong products, vacuum filtration is more commonly used for this purpose.

















