
Biogen Inc. is an American multinational biotechnology company based in Cambridge, Massachusetts, United States specializing in the discovery, development, and delivery of therapies for the treatment of neurological diseases to patients worldwide. Biogen operates in Argentina, Brazil, Canada, China, France, Germany, Hungary, India, Italy, Japan, Mexico, Netherlands, Poland, Sweden, and Switzerland.
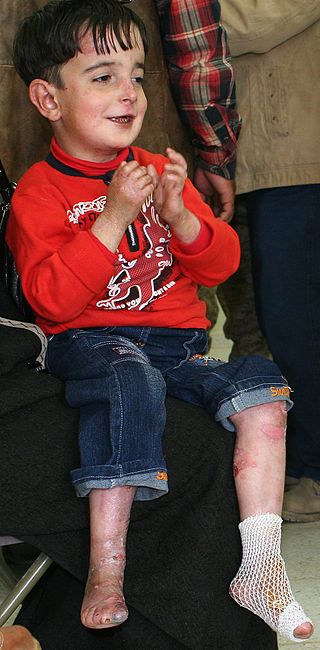
Epidermolysis bullosa (EB) is a group of rare medical conditions that result in easy blistering of the skin and mucous membranes. Blisters occur with minor trauma or friction and are painful. Its severity can range from mild to fatal. Inherited EB is a rare disease with a prevalence in the United States of 8.2 per million live births. Those with mild cases may not develop symptoms until they start to crawl or walk. Complications may include esophageal narrowing, squamous cell skin cancer, and the need for amputations.
DEBRA is the name of an international medical research charity dedicated to securing effective drug treatments and ultimately cures for every type of epidermolysis bullosa, with national groups in over 40 countries including in the United Kingdom and the United States.
Neurocrine Biosciences, Inc. is an American biopharmaceutical company founded in 1992. It is headquartered in San Diego, California, and led by CEO Kevin Gorman. Neurocrine develops treatments for neurological and endocrine-related diseases and disorders. In 2017, the company's drug valbenazine (Ingrezza) was approved in the US to treat adults with tardive dyskinesia (TD).

Epidermolysis bullosa dystrophica or dystrophic EB (DEB) is an inherited disease affecting the skin and other organs.

Genodermatosis is a hereditary skin disease with three inherited modes including single gene inheritance, multiple gene inheritance and chromosome inheritance. There are many different types of genodermatosis; the prevalence of genodermatosis ranges from 1 per 6000 people to 1 per 500,000 people. Genodermatosis has influence on the texture, color and structure of skin cuticle and connective tissue, specific lesion site and clinical manifestations on the body vary depending on the type. In the spite of the variety and complexity of genodermatosis, there are still some common methods that can help people diagnose. After diagnosis, different types of genodermatosis require different levels of therapy including interventions, nursing interventions and treatments. Among that, research of therapy for some new, complex and rare types are still in the developing stage. The impact of genodermatosis not only can be seen in body but also can be seen in all aspects of patients' life, including but not limited to psychological, family life, economic conditions and social activities. Accordingly, the patients need treatment, support and help in these areas.
Protein replacement therapy is a medical treatment that supplements or replaces a protein in patients in whom that particular protein is deficient or absent. There have been significant advances in this treatment. PRT is being tested in clinical trials with the diseases progeria and epidermolysis bullosa dystrophica as a potential treatment. For patients with epidermolysis bullosa dystrophica there have been promising results.
Junctional epidermolysis bullosa is a skin condition characterized by blister formation within the lamina lucida of the basement membrane zone.
A coma blister, or coma bullae, is a skin lesion or blister that typically arises due to pressure in an individual with impaired consciousness. They vary in size, ranging from 4 to 5 centimeters in diameter, and may appear hemorrhagic or blood filled. Coma blisters are usually found in the extremities and trunk. These types of blisters have been associated with the overdose of central nervous system (CNS) depressants especially barbiturates, but also tricyclic antidepressants, hypnotics, benzodiazepines, opiates, antipsychotics, and alcohol. However, studies have found that coma blisters are not caused by the toxicity of these drugs, but due to hypoxia and external pressure on the comatose individual's skin from being immobilized. Coma blisters have been frequently found on individuals who have overdosed on drugs, but have also been found on individuals with chronic kidney failure, hypercalcemia, diabetic ketoacidosis, and a variety of neurologic conditions. Coma blisters are more frequent in adults and less common among children as demonstrated by the few cases published in literature.
Teprotumumab, sold under the brand name Tepezza, is a medication used to treat adults with thyroid eye disease, a rare condition where the muscles and fatty tissues behind the eye become inflamed, causing the eyes to bulge outwards.
Bimagrumab (BYM338) is a human monoclonal antibody developed by Novartis to treat pathological muscle loss and weakness. It binds to and inhibits activin receptor type-2B.

Amicus Therapeutics, Inc. is a public American biopharmaceutical company based in Philadelphia, PA. The company went public in 2007 under the NASDAQ trading symbol FOLD. This followed a 2006 planned offering and subsequent withdrawal, which would have established the trading symbol as AMTX Prior to their IPO, Amicus was funded by a variety of venture capital firms including Radius Ventures, Canaan Partners and New Enterprise Associates.

Ionis Pharmaceuticals, Inc. is a biotechnology company based in Carlsbad, California, that specializes in discovering and developing RNA-targeted therapeutics. The company has three commercially approved medicines: Spinraza (Nusinersen), Tegsedi (Inotersen), and Waylivra (Volanesorsen), and has four drugs in pivotal studies: tominersen for Huntington's disease, tofersen for SOD1-ALS, AKCEA-APO(a)-LRx for cardiovascular disease, and AKCEA-TTR-LRx for all forms of TTR amyloidosis.

Migalastat, sold under the brand name Galafold, is a medication for the treatment of Fabry disease, a rare genetic disorder. It was developed by Amicus Therapeutics. The US Food and Drug Administration (FDA) granted it orphan drug status in 2004, and the European Commission followed in 2006. The European Medicines Agency's Committee for Medicinal Products for Human Use (CHMP) granted the drug a marketing approval under the name Galafold in May 2016.
Emapalumab, sold under the brand name Gamifant, is an anti-interferon-gamma (IFNγ) antibody medication used for the treatment of hemophagocytic lymphohistiocytosis (HLH), which has no cure.

CRISPR Therapeutics AG is a Swiss–American biotechnology company headquartered in Zug, Switzerland. It was one of the first companies formed to utilize the CRISPR gene editing platform to develop medicines for the treatment of various rare and common diseases. The company has approximately 500 employees and has offices in Zug, Switzerland, Boston, Massachusetts, San Francisco, California and London, United Kingdom. Its manufacturing facility in Framingham, Massachusetts won the Facilities of the Year Award (FOYA) award in 2022. The company’s lead program, exagamglogene autotemcel, or exa-cel, was granted regulatory approval by the US Food and Drug Administration (FDA) in December 2023.
Regenerative Medicine Advanced Therapy (RMAT) is a designation given by the Food and Drug Administration to drug candidates intended to treat serious or life-threatening conditions under the 21st Century Cures Act. A RMAT designation allows for accelerated approval based surrogate or intermediate endpoints.
Beremagene geperpavec, sold under the brand name Vyjuvek, is a gene therapy for the treatment of wounds. Beremagene geperpavec is the first approved gene therapy to use herpes-simplex virus type 1 as a vector. Beremagene geperpavec is a genetically modified herpes-simplex virus used to deliver normal copies of the COL7A1 gene to the wounds.

Birch triterpenes, sold under the brand name Filsuvez, is an extract of birch bark used as a topical medication for the treatment of epidermolysis bullosa. The active ingredients are triterpenes extracted from the outer bark of silver birch and downy birch.








