
A lithium-ion or Li-ion battery is a type of rechargeable battery that uses the reversible intercalation of Li+ ions into electronically conducting solids to store energy. In comparison with other commercial rechargeable batteries, Li-ion batteries are characterized by higher specific energy, higher energy density, higher energy efficiency, a longer cycle life, and a longer calendar life. Also noteworthy is a dramatic improvement in lithium-ion battery properties after their market introduction in 1991: over the following 30 years, their volumetric energy density increased threefold while their cost dropped tenfold. In late 2024 global demand passed 1 Terawatt-hour per year, while production capacity was more than twice that.

A rechargeable battery, storage battery, or secondary cell, is a type of electrical battery which can be charged, discharged into a load, and recharged many times, as opposed to a disposable or primary battery, which is supplied fully charged and discarded after use. It is composed of one or more electrochemical cells. The term "accumulator" is used as it accumulates and stores energy through a reversible electrochemical reaction. Rechargeable batteries are produced in many different shapes and sizes, ranging from button cells to megawatt systems connected to stabilize an electrical distribution network. Several different combinations of electrode materials and electrolytes are used, including lead–acid, zinc–air, nickel–cadmium (NiCd), nickel–metal hydride (NiMH), lithium-ion (Li-ion), lithium iron phosphate (LiFePO4), and lithium-ion polymer.

An alkaline battery is a type of primary battery where the electrolyte has a pH value above 7. Typically these batteries derive energy from the reaction between zinc metal and manganese dioxide.

A zinc–air battery is a metal–air electrochemical cell powered by the oxidation of zinc with oxygen from the air. During discharge, a mass of zinc particles forms a porous anode, which is saturated with an electrolyte. Oxygen from the air reacts at the cathode and forms hydroxyl ions which migrate into the zinc paste and form zincate, releasing electrons to travel to the cathode. The zincate decays into zinc oxide and water returns to the electrolyte. The water and hydroxyl from the anode are recycled at the cathode, so the water is not consumed. The reactions produce a theoretical voltage of 1.65 Volts, but is reduced to 1.35–1.4 V in available cells.

A zinc–carbon battery (or carbon zinc battery in U.S. English) is a dry cell primary battery that provides direct electric current from the electrochemical reaction between zinc (Zn) and manganese dioxide (MnO2) in the presence of an ammonium chloride (NH4Cl) electrolyte. It produces a voltage of about 1.5 volts between the zinc anode, which is typically constructed as a cylindrical container for the battery cell, and a carbon rod surrounded by a compound with a higher Standard electrode potential (positive polarity), known as the cathode, that collects the current from the manganese dioxide electrode. The name "zinc-carbon" is slightly misleading as it implies that carbon is acting as the oxidizing agent rather than the manganese dioxide.
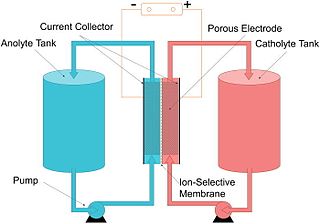
A flow battery, or redox flow battery, is a type of electrochemical cell where chemical energy is provided by two chemical components dissolved in liquids that are pumped through the system on separate sides of a membrane. Ion transfer inside the cell occurs across the membrane while the liquids circulate in their respective spaces.

A nickel–zinc battery is a type of rechargeable battery similar to nickel–cadmium batteries, but with a higher voltage of 1.6 V.

Molten-salt batteries are a class of battery that uses molten salts as an electrolyte and offers both a high energy density and a high power density. Traditional non-rechargeable thermal batteries can be stored in their solid state at room temperature for long periods of time before being activated by heating. Rechargeable liquid-metal batteries are used for industrial power backup, special electric vehiclesand for grid energy storage, to balance out intermittent renewable power sources such as solar panels and wind turbines.

A lithium-ion capacitor is a hybrid type of capacitor classified as a type of supercapacitor. It is called a hybrid because the anode is the same as those used in lithium-ion batteries and the cathode is the same as those used in supercapacitors. Activated carbon is typically used as the cathode. The anode of the LIC consists of carbon material which is often pre-doped with lithium ions. This pre-doping process lowers the potential of the anode and allows a relatively high output voltage compared to other supercapacitors.
Rechargeable lithium metal batteries are secondary lithium metal batteries. They have metallic lithium as a negative electrode. The high specific capacity of lithium metal, very low redox potential and low density make it the ideal negative material for high energy density battery technologies. Rechargeable lithium metal batteries can have a long run time due to the high charge density of lithium. Several companies and many academic research groups are currently researching and developing rechargeable lithium metal batteries as they are considered a leading pathway for development beyond lithium-ion batteries. Some rechargeable lithium metal batteries employ a liquid electrolyte and some employ a solid-state electrolyte.

An electric battery is a source of electric power consisting of one or more electrochemical cells with external connections for powering electrical devices. When a battery is supplying power, its positive terminal is the cathode and its negative terminal is the anode. The terminal marked negative is the source of electrons. When a battery is connected to an external electric load, those negatively charged electrons flow through the circuit and reach to the positive terminal, thus cause a redox reaction by attracting positively charged ions, cations. Thus converts high-energy reactants to lower-energy products, and the free-energy difference is delivered to the external circuit as electrical energy. Historically the term "battery" specifically referred to a device composed of multiple cells; however, the usage has evolved to include devices composed of a single cell.
The lithium–air battery (Li–air) is a metal–air electrochemical cell or battery chemistry that uses oxidation of lithium at the anode and reduction of oxygen at the cathode to induce a current flow.
A metal–air electrochemical cell is an electrochemical cell that uses an anode made from pure metal and an external cathode of ambient air, typically with an aqueous or aprotic electrolyte.
A potassium-ion battery or K-ion battery is a type of battery and analogue to lithium-ion batteries, using potassium ions for charge transfer instead of lithium ions.

Sodium-ion batteries (NIBs, SIBs, or Na-ion batteries) are several types of rechargeable batteries, which use sodium ions (Na+) as their charge carriers. In some cases, its working principle and cell construction are similar to those of lithium-ion battery (LIB) types, but it replaces lithium with sodium as the intercalating ion. Sodium belongs to the same group in the periodic table as lithium and thus has similar chemical properties. However, in some cases, such as aqueous batteries, SIBs can be quite different from LIBs.

A supercapacitor (SC), also called an ultracapacitor, is a high-capacity capacitor, with a capacitance value much higher than solid-state capacitors but with lower voltage limits. It bridges the gap between electrolytic capacitors and rechargeable batteries. It typically stores 10 to 100 times more energy per unit volume or mass than electrolytic capacitors, can accept and deliver charge much faster than batteries, and tolerates many more charge and discharge cycles than rechargeable batteries.
Aluminium-ion batteries (AIB) are a class of rechargeable battery in which aluminium ions serve as charge carriers. Aluminium can exchange three electrons per ion. This means that insertion of one Al3+ is equivalent to three Li+ ions. Thus, since the ionic radii of Al3+ (0.54 Å) and Li+ (0.76 Å) are similar, significantly higher numbers of electrons and Al3+ ions can be accepted by cathodes with little damage. Al has 50 times (23.5 megawatt-hours m-3) the energy density of Li-ion batteries and is even higher than coal.

Pseudocapacitance is the electrochemical storage of electricity in an electrochemical capacitor that occurs due to faradaic charge transfer originating from a very fast sequence of reversible faradaic redox, electrosorption or intercalation processes on the surface of suitable electrodes. Pseudocapacitance is accompanied by an electron charge-transfer between electrolyte and electrode coming from a de-solvated and adsorbed ion. One electron per charge unit is involved. The adsorbed ion has no chemical reaction with the atoms of the electrode since only a charge-transfer takes place. Supercapacitors that rely primarily on pseudocapacitance are sometimes called pseudocapacitors.
Research in lithium-ion batteries has produced many proposed refinements of lithium-ion batteries. Areas of research interest have focused on improving energy density, safety, rate capability, cycle durability, flexibility, and reducing cost.
Magnesium batteries are batteries that utilize magnesium cations as charge carriers and possibly in the anode in electrochemical cells. Both non-rechargeable primary cell and rechargeable secondary cell chemistries have been investigated. Magnesium primary cell batteries have been commercialised and have found use as reserve and general use batteries.













