
A bacteriophage, also known informally as a phage, is a virus that infects and replicates within bacteria and archaea. The term was derived from "bacteria" and the Greek φαγεῖν, meaning "to devour". Bacteriophages are composed of proteins that encapsulate a DNA or RNA genome, and may have structures that are either simple or elaborate. Their genomes may encode as few as four genes and as many as hundreds of genes. Phages replicate within the bacterium following the injection of their genome into its cytoplasm.

Cholera is an infection of the small intestine by some strains of the bacterium Vibrio cholerae. Symptoms may range from none, to mild, to severe. The classic symptom is large amounts of watery diarrhea that lasts a few days. Vomiting and muscle cramps may also occur. Diarrhea can be so severe that it leads within hours to severe dehydration and electrolyte imbalance. This may result in sunken eyes, cold skin, decreased skin elasticity, and wrinkling of the hands and feet. Dehydration can cause the skin to turn bluish. Symptoms start two hours to five days after exposure.

Vibrio cholerae is a species of Gram-negative, facultative anaerobe and comma-shaped bacteria. The bacteria naturally live in brackish or saltwater where they attach themselves easily to the chitin-containing shells of crabs, shrimps, and other shellfish. Some strains of V. cholerae are pathogenic to humans and cause a deadly disease cholera, which can be derived from the consumption of undercooked or raw marine life species.
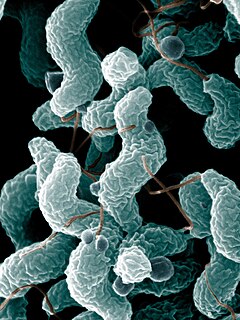
Campylobacter is a genus of Gram-negative bacteria. Campylobacter typically appear comma- or s-shaped, and are motile. Some Campylobacter species can infect humans, sometimes causing campylobacteriosis, a diarrhoeal disease in humans. Campylobacteriosis is usually self-limiting and antimicrobial treatment is often not required, except in severe cases or immunocompromised patients. The most known source for Campylobacter is poultry, but due to their diverse natural reservoir, Campylobacter spp. can also be transmitted via water. Other known sources of Campylobacter infections include food products, such as unpasteurised milk and contaminated fresh produce. Sometimes the source of infection can be direct contact with infected animals, which often carry Campylobacter asymptomatically. At least a dozen species of Campylobacter have been implicated in human disease, with C. jejuni (80–90%) and C. coli (5-10%) being the most common. C. jejuni is recognized as one of the main causes of bacterial foodborne disease in many developed countries. It is the number one cause of bacterial gastroentritis in Europe, with over 246,000 cases confirmed annually. C. jejuni infection can also cause bacteremia in immunocompromised individuals, while C. lari is a known cause of recurrent diarrhea in children. C. fetus can cause spontaneous abortions in cattle and sheep, and is an opportunistic pathogen in humans.

Vibrio is a genus of Gram-negative bacteria, possessing a curved-rod (comma) shape, several species of which can cause foodborne infection, usually associated with eating undercooked seafood. Typically found in salt water, Vibrio species are facultative anaerobes that test positive for oxidase and do not form spores. All members of the genus are motile. They are able to have polar or lateral flagellum with or without sheaths. Vibrio species typically possess two chromosomes, which is unusual for bacteria. Each chromosome has a distinct and independent origin of replication, and are conserved together over time in the genus. Recent phylogenies have been constructed based on a suite of genes.

A prophage is a bacteriophage genome inserted and integrated into the circular bacterial DNA chromosome or exists as an extrachromosomal plasmid. This is a latent form of a phage, in which the viral genes are present in the bacterium without causing disruption of the bacterial cell. Pro means "before", so, prophage means the stage of a virus in the form of genome inserted into host DNA before being activated inside the host.
Pathogenicity islands (PAIs), as termed in 1990, are a distinct class of genomic islands acquired by microorganisms through horizontal gene transfer. Pathogenicity islands are found in both animal and plant pathogens. Additionally, PAIs are found in both gram-positive and gram-negative bacteria. They are transferred through horizontal gene transfer events such as transfer by a plasmid, phage, or conjugative transposon. Therefore, PAIs contribute to microorganisms' ability to evolve.
G. Balakrish Nair is an Indian microbiologist. At present, he is the Ag. Regional Adviser, Research Policy and Cooperation Unit, Department of Communicable Diseases, World Health Organization. Before joining WHO, he was the executive director of Translational Health Science and Technology Institute (THSTI), Faridabad, NCR, India. Before joining THSTI, he was working in NICED as the director. He has also served as the director of Laboratory Sciences Division at the International Center for Diarrhoeal Diseases Research,, Dhaka, Bangladesh.

Filamentous bacteriophage is a family of viruses (Inoviridae) that infect bacteria. The phages are named for their filamentous shape, a worm-like chain, about 6 nm in diameter and about 1000-2000 nm long. The coat of the virion comprises five types of viral protein, which are located during phage assembly in the inner membrane of the host bacteria, and are added to the nascent virion as it extrudes through the membrane. The simplicity of this family makes it an attractive model system to study fundamental aspects of molecular biology, and it has also proven useful as a tool in immunology and nanotechnology.

Lysogeny, or the lysogenic cycle, is one of two cycles of viral reproduction. Lysogeny is characterized by integration of the bacteriophage nucleic acid into the host bacterium's genome or formation of a circular replicon in the bacterial cytoplasm. In this condition the bacterium continues to live and reproduce normally. The genetic material of the bacteriophage, called a prophage, can be transmitted to daughter cells at each subsequent cell division, and later events can release it, causing proliferation of new phages via the lytic cycle. Lysogenic cycles can also occur in eukaryotes, although the method of DNA incorporation is not fully understood. For instance the AIDS viruses can either infect humans lytically, or lay dormant (lysogenic) as part of the infected cells' genome, keeping the ability to return to lysis at a later time. The rest of this article is about lysogeny in bacterial hosts.

icddr,b is an international health research organisation located in Dhaka, Bangladesh. Dedicated to saving lives through research and treatment, icddr,b addresses some of the most critical health concerns facing the world today, ranging from improving neonatal survival to HIV/AIDS. In collaboration with academic and research institutions over the world, icddr,b conducts research, training and extension activities, as well as programme-based activities, to develop and share knowledge for global lifesaving solutions.
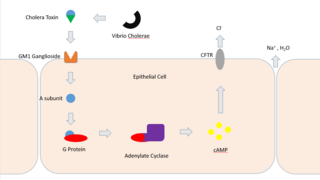
Cholera toxin is AB5 multimeric protein complex secreted by the bacterium Vibrio cholerae. CTX is responsible for the massive, watery diarrhea characteristic of cholera infection. It is a member of the Heat-labile enterotoxin family.
The CTXφ bacteriophage is a filamentous bacteriophage. It is a positive-strand DNA virus with single-stranded DNA (ssDNA).
Firdausi Qadri is a Bangladeshi scientist with specialization in immunology and infectious disease research. She has worked over 25 years on the development of vaccines for cholera and has expertise on other infectious disease like ETEC, Typhoid, Helicobacter pylori, rotavirus, etc. Currently, she is working as a director for Centre for Vaccine Sciences of International Centre for Diarrhoeal Disease and Research, Bangladesh (icddr,b). She also serves as chairperson of the Institute for developing Science and Health initiatives. Her scientific achievements lie in enteric infections and vaccines including Vibrio cholerae and enterotoxigenic Escherichia coli—major causes of severe diarrhea. She has also focused on studying the immune response in H.pylori infected people in Bangladesh and the responses in patients with typhoid fever as well as vaccinees.

Edward Thomas Ryan is an American microbiologist, immunologist, and physician at Harvard University and Massachusetts General Hospital. Ryan served as President of the American Society of Tropical Medicine and Hygiene from 2009 to 2010. Ryan is Professor of Immunology and Infectious Diseases at the Harvard T.H. Chan School of Public Health, Professor of Medicine at Harvard Medical School, and Director of Global Infectious Diseases at the Massachusetts General Hospital. Ryan's research and clinical focus has been on infectious diseases associated with residing in, immigrating from, or traveling through resource-limited areas. Ryan is a Fellow of the American Society of Microbiology, the American Society of Tropical Medicine and Hygiene, the American College of Physicians, and the Infectious Diseases Society of America.
The type VI secretion system (T6SS) is molecular machine used by a wide range of Gram-negative bacterial species to transport proteins from the interior of a bacterial cell across the cellular envelope into an adjacent target cell. While often reported that the T6SS was discovered in 2006 by researchers studying the causative agent of cholera, Vibrio cholerae, the first study demonstrating that T6SS genes encode a protein export apparatus was actually published in 2004, in a study of protein secretion by the fish pathogen Edwardsiella tarda.
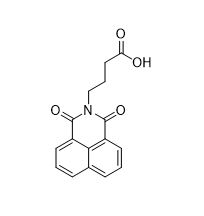
Virstatin is a small molecule that inhibits the activity of the cholera protein, ToxT.
Bacterial phylodynamics is the study of immunology, epidemiology, and phylogenetics of bacterial pathogens to better understand the evolutionary role of these pathogens. Phylodynamic analysis includes analyzing genetic diversity, natural selection, and population dynamics of infectious disease pathogen phylogenies during pandemics and studying intra-host evolution of viruses. Phylodynamics combines the study of phylogenetic analysis, ecological, and evolutionary processes to better understand of the mechanisms that drive spatiotemportal incidence and phylogenetic patterns of bacterial pathogens. Bacterial phylodynamics uses genome-wide single-nucleotide polymorphisms (SNP) in order to better understand the evolutionary mechanism of bacterial pathogens. Many phylodynamic studies have been performed on viruses, specifically RNA viruses which have high mutation rates. The field of bacterial phylodynamics has increased substantially due to the advancement of next-generation sequencing and the amount of data available.
John Mekalanos is a microbiologist who is primarily known for leading one of the first teams that reported the discovery of the type VI secretion system as well as his work on the pathogenicity of the bacterial species Vibrio cholerae, its toxin, and its secretion systems. Since 1998, he has been a member of the National Academy of Sciences.

Melanie Blokesch is a German microbiologist. Her research focuses on Vibrio cholerae, the bacterium causing cholera. She is a professor of life sciences at École Polytechnique Fédérale de Lausanne (EPFL), where she heads the Laboratory of Molecular Microbiology.













