Bevacizumab, sold under the brand name Avastin among others, is a monoclonal antibody medication used to treat a number of types of cancers and a specific eye disease. For cancer, it is given by slow injection into a vein (intravenous) and used for colon cancer, lung cancer, ovarian cancer, glioblastoma, hepatocellular carcinoma, and renal-cell carcinoma. In many of these diseases it is used as a first-line therapy. For age-related macular degeneration it is given by injection into the eye (intravitreal).

Breast Cancer Awareness Month (BCAM), also referred to in the United States as National Breast Cancer Awareness Month (NBCAM), is an annual international health campaign organized by major breast cancer charities every October to increase awareness of the disease and raise funds for research into its cause, prevention, diagnosis, treatment, and cure.
Triple-negative breast cancer (TNBC) is any breast cancer that either lacks or shows low levels of estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2) overexpression and/or gene amplification. Triple-negative is sometimes used as a surrogate term for basal-like.
The Triple Negative Breast Cancer Foundation is a nonprofit organization dedicated to raising awareness of triple negative breast cancer. The foundation supports scientists and researchers in their efforts to determine the definitive causes of triple negative breast cancer so that effective detection, diagnosis, prevention, and treatment can be pursued and achieved.

The Breast Cancer Research Foundation (BCRF) is an independent, not-for-profit organization which has raised $569.4 million to support clinical and translational research on breast cancer at medical institutions in the United States and abroad. BCRF currently funds over 255 researchers in 14 countries.

The Prostate Cancer Foundation (PCF), headquartered in Santa Monica, California, funds research into the prevention and cure of prostate cancer.
Sipuleucel-T, sold under the brand name Provenge, developed by Dendreon Pharmaceuticals, LLC, is a cell-based cancer immunotherapy for prostate cancer (CaP). It is an autologous cellular immunotherapy.

Trastuzumab emtansine, sold under the brand name Kadcyla, is an antibody-drug conjugate consisting of the humanized monoclonal antibody trastuzumab (Herceptin) covalently linked to the cytotoxic agent DM1. Trastuzumab alone stops growth of cancer cells by binding to the HER2 receptor, whereas trastuzumab emtansine undergoes receptor-mediated internalization into cells, is catabolized in lysosomes where DM1-containing catabolites are released and subsequently bind tubulin to cause mitotic arrest and cell death. Trastuzumab binding to HER2 prevents homodimerization or heterodimerization (HER2/HER3) of the receptor, ultimately inhibiting the activation of MAPK and PI3K/AKT cellular signalling pathways. Because the monoclonal antibody targets HER2, and HER2 is only over-expressed in cancer cells, the conjugate delivers the cytotoxic agent DM1 specifically to tumor cells. The conjugate is abbreviated T-DM1.
High-dose chemotherapy and bone marrow transplant (HDC/BMT), also high-dose chemotherapy with autologous bone marrow transplant, was an ineffective treatment regimen for metastatic breast cancer, and later high-risk breast cancer, that was considered promising during the 1980s and 1990s. With an overall idea that more is better, this process involved taking cells from the person's bone marrow to store in a lab, then to give such high doses of chemotherapy drugs that the remaining bone marrow was destroyed, and then to inject the cells taken earlier back into the body as replacement. It was ultimately determined to be no more effective than normal treatment, and to have significantly higher side effects, including treatment-related death.

Pembrolizumab, sold under the brand name Keytruda, is a humanized antibody, more specifically a PD-1 Inhibitor, used in cancer immunotherapy that treats melanoma, lung cancer, head and neck cancer, Hodgkin lymphoma, stomach cancer, cervical cancer, and certain types of breast cancer. It is administered by slow intravenous injection.

Palbociclib, sold under the brand name Ibrance among others, is a medication developed by Pfizer for the treatment of HR-positive and HER2-negative breast cancer. It is a selective inhibitor of the cyclin-dependent kinases CDK4 and CDK6. Palbociclib was the first CDK4/6 inhibitor to be approved as a cancer therapy.

Atezolizumab, sold under the brand name Tecentriq among others, is a monoclonal antibody medication used to treat urothelial carcinoma, non-small cell lung cancer (NSCLC), small cell lung cancer (SCLC), hepatocellular carcinoma and alveolar soft part sarcoma, but discontinued for use in triple-negative breast cancer (TNBC). It is a fully humanized, engineered monoclonal antibody of IgG1 isotype against the protein programmed cell death-ligand 1 (PD-L1).

Sacituzumab govitecan, sold under the brand name Trodelvy by Gilead Sciences, is a Trop-2-directed antibody and topoisomerase inhibitor drug conjugate used for the treatment of metastatic triple-negative breast cancer and metastatic urothelial cancer.

Buparlisib is an experimental anti-cancer medication. It is a small molecule orally-available pan-class I phosphoinositide 3-kinase (PI3K) inhibitor. Buparlisib was under investigation as a treatment for advanced breast cancer but was abandoned due to negative results. It is still under investigation as a potential treatment for head and neck squamous cell carcinoma (HNSCC).
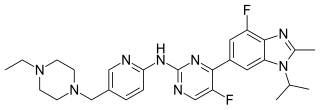
Abemaciclib, sold under the brand name Verzenio among others, is a medication for the treatment of advanced or metastatic breast cancers. It was developed by Eli Lilly and it acts as a CDK inhibitor selective for CDK4 and CDK6.

Elacestrant, sold under the brand name Orserdu, is a selective estrogen receptor degrader (SERD) used in the treatment of breast cancer. It is taken by mouth.
Project Pink Blue, registered as Health & Psychological Trust Centre is a cancer nonprofit engaged in raising cancer awareness, patient navigation, advocacy and free breast and cervical cancer screening for women living in poverty. The organization launched Nigeria's first patient navigation in 2015 and a toll- free telephone centre 08000CANCER in 2016 Project PINK BLUE won the SPARC Metastatic Breast Cancer challenge grant by Union for International Cancer Control and Pfizer Oncology in Lisbon, Portugal.
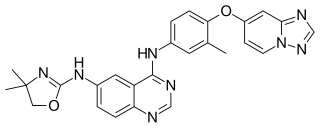
Tucatinib, sold under the brand name Tukysa, is an anticancer medication used for the treatment of HER2-positive breast cancer. It is a small molecule inhibitor of HER2. It was developed by Array BioPharma and licensed to Cascadian Therapeutics.
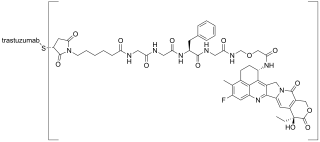
Trastuzumab deruxtecan, sold under the brand name Enhertu, is an antibody-drug conjugate consisting of the humanized monoclonal antibody trastuzumab (Herceptin) covalently linked to the topoisomerase I inhibitor deruxtecan. It is licensed for the treatment of breast cancer or gastric or gastroesophageal adenocarcinoma. Trastuzumab binds to and blocks signaling through epidermal growth factor receptor 2 (HER2/neu) on cancers that rely on it for growth. Additionally, once bound to HER2 receptors, the antibody is internalized by the cell, carrying the bound deruxtecan along with it, where it interferes with the cell's ability to make DNA structural changes and replicate its DNA during cell division, leading to DNA damage when the cell attempts to replicate itself, destroying the cell.














