Related Research Articles
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic table: carbon is above it; and germanium, tin, lead, and flerovium are below it. It is relatively unreactive.
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula SiO2, most commonly found in nature as quartz In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and most abundant families of materials, existing as a compound of several minerals and as a synthetic product. Notable examples include fused quartz, fumed silica, silica gel, opal and aerogels. It is used in structural materials, microelectronics, and as components in the food and pharmaceutical industries.

Thermal insulation is the reduction of heat transfer between objects in thermal contact or in range of radiative influence. Thermal insulation can be achieved with specially engineered methods or processes, as well as with suitable object shapes and materials.

Silica gel is an amorphous and porous form of silicon dioxide (silica), consisting of an irregular tridimensional framework of alternating silicon and oxygen atoms with nanometer-scale voids and pores. The voids may contain water or some other liquids, or may be filled by gas or vacuum. In the last case, the material is properly called silica xerogel.
Silicon tetrachloride or tetrachlorosilane is the inorganic compound with the formula SiCl4. It is a colourless volatile liquid that fumes in air. It is used to produce high purity silicon and silica for commercial applications. It's part of the chlorosilane family.

Silica fume, also known as microsilica, is an amorphous (non-crystalline) polymorph of silicon dioxide, silica. It is an ultrafine powder collected as a by-product of the silicon and ferrosilicon alloy production and consists of spherical particles with an average particle diameter of 150 nm. The main field of application is as pozzolanic material for high performance concrete.
In materials science, the sol–gel process is a method for producing solid materials from small molecules. The method is used for the fabrication of metal oxides, especially the oxides of silicon (Si) and titanium (Ti). The process involves conversion of monomers into a colloidal solution (sol) that acts as the precursor for an integrated network of either discrete particles or network polymers. Typical precursors are metal alkoxides. Sol-gel process is used to produce ceramic nanoparticles.
Samuel Stephens Kistler was an American scientist and chemical engineer, best known as the inventor of aerogels, one of the lightest known solid materials.

Stardust@home is a citizen science project that encourages volunteers to search images for tiny interstellar dust impacts. The project began providing data for analysis on August 1, 2006.

Peter Tsou is a principal science staff member at the Jet Propulsion Laboratory (JPL) of the California Institute of Technology, where he has worked for the past 34 years. Dr. Tsou's research is focused on using aerogel for space exploration.
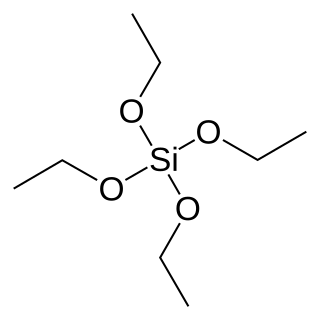
Tetraethyl orthosilicate, formally named tetraethoxysilane (TEOS), ethyl silicate is the organic chemical compound with the formula Si(OC2H5)4. TEOS is a colorless liquid. It degrades in water. TEOS is the ethyl ester of orthosilicic acid, Si(OH)4. It is the most prevalent alkoxide of silicon.
Silicon monoxide is the chemical compound with the formula SiO where silicon is present in the oxidation state +2. In the vapour phase, it is a diatomic molecule. It has been detected in stellar objects and has been described as the most common oxide of silicon in the universe.
The Stöber process is a chemical process used to prepare silica particles of controllable and uniform size for applications in materials science. It was pioneering when it was reported by Werner Stöber and his team in 1968, and remains today the most widely used wet chemistry synthetic approach to silica nanoparticles. It is an example of a sol-gel process wherein a molecular precursor is first reacted with water in an alcoholic solution, the resulting molecules then joining together to build larger structures. The reaction produces silica particles with diameters ranging from 50 to 2000 nm, depending on conditions. The process has been actively researched since its discovery, including efforts to understand its kinetics and mechanism – a particle aggregation model was found to be a better fit for the experimental data than the initially hypothesized LaMer model. The newly acquired understanding has enabled researchers to exert a high degree of control over particle size and distribution and to fine-tune the physical properties of the resulting material in order to suit intended applications.
Ultralight materials are solids with a density of less than 10 mg/cm3, including silica aerogels, carbon nanotube aerogels, aerographite, metallic foams, polymeric foams, and metallic microlattices. The density of air is about 1.275 mg/cm3, which means that the air in the pores contributes significantly to the density of these materials in atmospheric conditions. They can be classified by production method as aerogels, stochastic foams, and structured cellular materials.
Aerographene or graphene aerogel is, as of April 2020, the least dense solid known, at 160 g/m3, less than helium. It is approximately 7.5 times less dense than air. Note that the cited density does not include the weight of the air incorporated in the structure: it does not float in air. It was developed at Zhejiang University. The material reportedly can be produced at the scale of cubic meters.

In chemistry, orthosilicate is the anion SiO4−
4, or any of its salts and esters. It is one of the silicate anions. It is occasionally called the silicon tetroxide anion or group.

The Tanpopo mission is an orbital astrobiology experiment investigating the potential interplanetary transfer of life, organic compounds, and possible terrestrial particles in the low Earth orbit. The purpose is to assess the panspermia hypothesis and the possibility of natural interplanetary transport of microbial life as well as prebiotic organic compounds.
Frances Irene Mazze Hurwitz is an American materials research engineer at NASA Glenn Center, Cleveland, Ohio. Hurwitz is known for her work on heat-resistant materials, aerogels, Space Shuttle Columbia Accident Investigation and Shuttle Return to Flight. Hurwitz studied at Harpur College -SUNY Binghamton, Syracuse University, and Case Western Reserve University. She has been and continues to be instrumental in the development of aerogels used at NASA and in developing and testing new aerospace materials.

Aerogels are a class of synthetic porous ultralight material derived from a gel, in which the liquid component for the gel has been replaced with a gas, without significant collapse of the gel structure. The result is a solid with extremely low density and extremely low thermal conductivity. Aerogels can be made from a variety of chemical compounds. Silica aerogels feel like fragile expanded polystyrene to the touch, while some polymer-based aerogels feel like rigid foams.
In chemistry, a silicic acid is any chemical compound containing the element silicon attached to oxide and hydroxyl groups, with the general formula [H2xSiOx+2]n or, equivalently, [SiOx(OH)4-2x]n. Orthosilicic acid is a representative example. Silicic acids are rarely observed in isolation, but are thought to exist in aqueous solutions, including seawater, and play a role in biomineralization. They are typically colorless weak acids that are sparingly soluble in water. Like the silicate anions, which are their better known conjugate bases, silicic acids are proposed to be oligomeric or polymeric. No simple silicic acid has ever been identified, since these species being primarily of theoretical interest.
References
- ↑ "Production of Silica Gels: Alkoxide Method". aerogel.org.