
Niacinamide or nicotinamide is a form of vitamin B3 found in food and used as a dietary supplement and medication. As a supplement, it is used orally (swallowed by mouth) to prevent and treat pellagra (niacin deficiency). While nicotinic acid (niacin) may be used for this purpose, niacinamide has the benefit of not causing skin flushing. As a cream, it is used to treat acne, and has been observed in clinical studies to improve the appearance of aging skin by reducing hyperpigmentation and redness. It is a water-soluble vitamin. Niacinamide is the supplement name, while nicotinamide is the scientific name.

Nicotinamide adenine dinucleotide (NAD) is a coenzyme central to metabolism. Found in all living cells, NAD is called a dinucleotide because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine nucleobase and the other, nicotinamide. NAD exists in two forms: an oxidized and reduced form, abbreviated as NAD+ and NADH (H for hydrogen), respectively.
A diol is a chemical compound containing two hydroxyl groups. An aliphatic diol may also be called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified. They are used as protecting groups of carbonyl groups, making them essential in synthesis of organic chemistry.

A cofactor is a non-protein chemical compound or metallic ion that is required for an enzyme's role as a catalyst. Cofactors can be considered "helper molecules" that assist in biochemical transformations. The rates at which these happen are characterized in an area of study called enzyme kinetics. Cofactors typically differ from ligands in that they often derive their function by remaining bound.
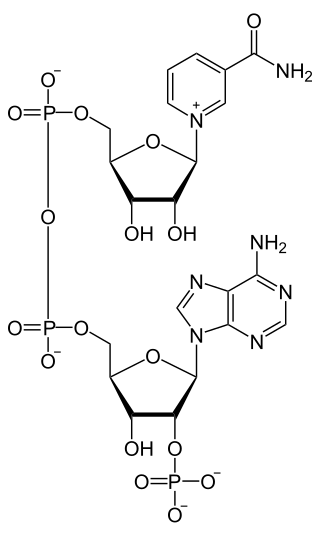
Nicotinamide adenine dinucleotide phosphate, abbreviated NADP+ or, in older notation, TPN (triphosphopyridine nucleotide), is a cofactor used in anabolic reactions, such as the Calvin cycle and lipid and nucleic acid syntheses, which require NADPH as a reducing agent ('hydrogen source'). NADPH is the reduced form, whereas NADP+ is the oxidized form. NADP+ is used by all forms of cellular life.

In biochemistry, flavin adenine dinucleotide (FAD) is a redox-active coenzyme associated with various proteins, which is involved with several enzymatic reactions in metabolism. A flavoprotein is a protein that contains a flavin group, which may be in the form of FAD or flavin mononucleotide (FMN). Many flavoproteins are known: components of the succinate dehydrogenase complex, α-ketoglutarate dehydrogenase, and a component of the pyruvate dehydrogenase complex.
The Baeyer–Villiger oxidation is an organic reaction that forms an ester from a ketone or a lactone from a cyclic ketone, using peroxyacids or peroxides as the oxidant. The reaction is named after Adolf von Baeyer and Victor Villiger who first reported the reaction in 1899.

Biocatalysis refers to the use of living (biological) systems or their parts to speed up (catalyze) chemical reactions. In biocatalytic processes, natural catalysts, such as enzymes, perform chemical transformations on organic compounds. Both enzymes that have been more or less isolated and enzymes still residing inside living cells are employed for this task. Modern biotechnology, specifically directed evolution, has made the production of modified or non-natural enzymes possible. This has enabled the development of enzymes that can catalyze novel small molecule transformations that may be difficult or impossible using classical synthetic organic chemistry. Utilizing natural or modified enzymes to perform organic synthesis is termed chemoenzymatic synthesis; the reactions performed by the enzyme are classified as chemoenzymatic reactions.
The branched-chain α-ketoacid dehydrogenase complex is a multi-subunit complex of enzymes that is found on the mitochondrial inner membrane. This enzyme complex catalyzes the oxidative decarboxylation of branched, short-chain alpha-ketoacids. BCKDC is a member of the mitochondrial α-ketoacid dehydrogenase complex family, which also includes pyruvate dehydrogenase and alpha-ketoglutarate dehydrogenase, key enzymes that function in the Krebs cycle.
Transmetalation (alt. spelling: transmetallation) is a type of organometallic reaction that involves the transfer of ligands from one metal to another. It has the general form:
In chemistry, a (redox) non-innocent ligand is a ligand in a metal complex where the oxidation state is not clear. Typically, complexes containing non-innocent ligands are redox active at mild potentials. The concept assumes that redox reactions in metal complexes are either metal or ligand localized, which is a simplification, albeit a useful one.

In enzymology, a NADH peroxidase (EC 1.11.1.1) is an enzyme that catalyzes the chemical reaction
Azobenzene reductase also known as azoreductase (EC 1.7.1.6) is an enzyme that catalyzes the chemical reaction:

A selenenic acid is an organoselenium compound and an oxoacid with the general formula RSeOH, where R ≠ H. It is the first member of the family of organoselenium oxoacids, which also include seleninic acids and selenonic acids, which are RSeO2H and RSeO3H, respectively. Selenenic acids derived from selenoenzymes are thought to be responsible for the antioxidant activity of these enzymes. This functional group is sometimes called SeO-selenoperoxol.
Nicotinamide cofactor analogues (mNADs), also called nicotinamide coenzyme biomimetics (NCBs), are artificial compounds that mimic the natural nicotinamide adenine dinucleotide cofactors in structure, to explore a mechanism or be used in biocatalysis or other applications. These nicotinamide cofactor mimics generally retain the nicotinamide moiety with varying substituents.
Mycofactocin (MFT) is a family of small molecules derived from a peptide of the type known as RiPP (ribosomally synthesized and post-translationally modified peptides), naturally occurring in many types of Mycobacterium. It was discovered in a bioinformatics study in 2011. All mycofactocins share a precursor in the form of premycofactocin (PMFT); they differ by the cellulose tail added. Being redox active, both PMFT and MFT have an oxidized dione (mycofactocinone) form and a reduced diol (mycofactocinol) form, respectively termed PMFTH2 and MFTH2.
Morphinone reductase is an enzyme which catalyzes the NADH-dependent saturation of the carbon-carbon double bond of morphinone and codeinone, yielding hydromorphone and hydrocodone respectively. This saturation reaction is assisted by a FMN cofactor and the enzyme is a member of the α/β-barrel flavoprotein family. The sequence of the enzyme has been obtained from bacteria Pseudomonas putida M10 and has been successfully expressed in yeast and other bacterial species. The enzyme is reported to harbor high sequence and structural similarity to the Old Yellow Enzyme, a large group of flavin-dependent redox biocatalysts of yeast species, and an oestrogen-binding protein of Candida albicans. The enzyme has demonstrated value in biosynthesis of semi-opiate drugs in microorganisms, expanding the chemical diversity of BIA biosynthesis.

Marco Wilhelmus Fraaije is a Dutch scientist whose research concerns enzymology of redox enzymes, enzyme discovery & engineering and biocatalysis at the Groningen Biomolecular Sciences and Biotechnology Institute (GBB) at the University of Groningen.
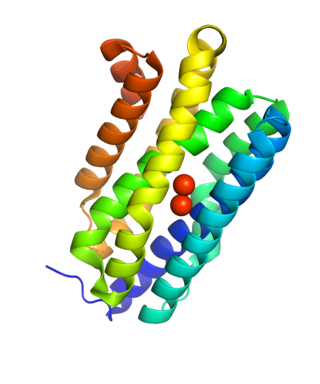
Aldehyde deformylating oxygenases (ADO) (EC 4.1.99.5) are a family of enzymes which catalyze the oxygenation of medium and long chain aldehydes to alkanes via the removal of a carbonyl group as formate.

Selin Kara is a Turkish-born chemist and biotechnologist. She is currently a full professor and head of Industrial Biotechnology section at Aarhus University. She studies biocatalysis and has been recognized for her work about deep eutectic solvents and her research regarding cofactor regeneration in biotransformations.












