This article provides insufficient context for those unfamiliar with the subject.(October 2009) (Learn how and when to remove this template message) |
The outer mitochondrial membrane is made up of two essential proteins, Tom40 and Sam50.
This article provides insufficient context for those unfamiliar with the subject.(October 2009) (Learn how and when to remove this template message) |
The outer mitochondrial membrane is made up of two essential proteins, Tom40 and Sam50.
Tom40 is a protein import pore required for the import of precursor proteins across the outer mitochondrial membrane, and it makes up part of the translocase of the outer membrane.
Sam50 is a subunit of the sorting and assembly machinery (SAM) of the outer membrane.
The sorting and assembly machinery is a protein complex, that operates after the translocase of the outer membrane, to mediate insertion of beta barrel proteins into the outer mitochondrial membrane. [1]
The sorting and assembly machinery is made up of three subunits, Sam35, Sam37, and Sam50, of which Sam50 is embedded within the outer mitochondrial membrane. [2]
Both Sam35 and Sam37 are located on the cytosolic face of the SAM complex are peripheral membrane proteins that are not essential for survival. [3]
Sam35 is believed to bind to substrate proteins, while Sam37 acts after Sam35 to stimulate release of the substrate protein from the SAM complex. [3]
The sorting and assembly machinery is required for the assembly of beta barrel proteins, this includes proteins such as the Tom40 import pore and porin.
Like all mitochondrial proteins, beta barrel proteins are transported into the intermembrane space of mitochondria via the translocase of the outer membrane.
Following import, beta barrel proteins are transported to the SAM complex by chaperone complexes, formed by assembly of the small Tim proteins. [3]
The complex formed by the three sorting and assembly machinery subunits (SAM core complex), is responsible for the integration of beta barrel proteins into the outer mitochondrial membrane. [3]
Mdm10 is another mitochondrial membrane protein, that is responsible for maintaining mitochondrial morphology and distribution. It has been found to interact with the SAM core complex, and may play a role in assembling Tom40 into the translocase of the outer membrane.
Protein targeting or protein sorting is the biological mechanism by which proteins are transported to their appropriate destinations in the cell or outside it. Proteins can be targeted to the inner space of an organelle, different intracellular membranes, plasma membrane, or to the exterior of the cell via secretion. This delivery process is carried out based on information contained in the protein itself. Correct sorting is crucial for the cell; errors can lead to diseases.

The intermembrane space (IMS) is the space occurring between or involving two or more membranes. In cell biology, it is most commonly described as the region between the inner membrane and the outer membrane of a mitochondrion or a chloroplast. It also refers to the space between the inner and outer nuclear membranes of the nuclear envelope, but is often called the perinuclear space. The IMS of mitochondria plays a crucial role in coordinating a variety of cellular activities, such as regulation of respiration and metabolic functions. Unlike the IMS of the mitochondria, the IMS of the chloroplast does not seem to have any obvious function.

The bacterial outer membrane is found in gram-negative bacteria. Its composition is distinct from that of the inner cytoplasmic cell membrane - among other things, the outer leaflet of the outer membrane of many gram-negative bacteria includes a complex lipopolysaccharide whose lipid portion acts as an endotoxin - and in some bacteria such as E. coli it is linked to the cell's peptidoglycan by Braun's lipoprotein.

The inner mitochondrial membrane (IMM) is the mitochondrial membrane which separates the mitochondrial matrix from the intermembrane space.
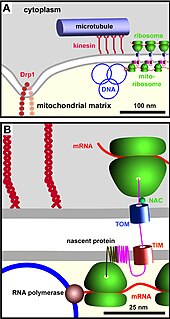
The TIM/TOM complex is a protein complex in cellular biochemistry which translocates proteins produced from nuclear DNA through the mitochondrial membrane for use in oxidative phosphorylation. Only 13 proteins necessary for a mitochondrion are actually coded in mitochondrial DNA.

Mitochondrial import inner membrane translocase subunit Tim8 A, also known as Deafness-dystonia peptide or protein is an enzyme that in humans is encoded by the TIMM8A gene. This translocase has similarity to yeast mitochondrial proteins that are involved in the import of metabolite transporters from the cytoplasm into the mitochondrial inner membrane. The gene is mutated in Deafness-dystonia syndrome and it is postulated that MTS/DFN-1 is a mitochondrial disease caused by a defective mitochondrial protein import system.

Mitochondrial import receptor subunit TOM20 homolog is a protein that in humans is encoded by the TOMM20 gene.

Mitochondrial import receptor subunit TOM22 homolog is a protein that in humans is encoded by the TOMM22 gene.

Mitochondrial import inner membrane translocase subunit Tim13 is an enzyme that in humans is encoded by the TIMM13 gene.

The translocase of the outer membrane (TOM) is a complex of proteins found in the outer mitochondrial membrane of the mitochondria. It allows movement of proteins through this barrier and into the intermembrane space of the mitochondrion. Most of the proteins needed for mitochondrial function are encoded by the nucleus of the cell. The outer membrane of the mitochondrion is impermeable to large molecules greater than 5000 Daltons. The TOM works in conjunction with the translocase of the inner membrane (TIM) to translocate proteins into the mitochondrion. Many of the proteins in the TOM complex, such as TOMM22, were first identified in Neurospora crassa and Saccharomyces cerevisiae.

Mitochondrial import receptor subunit TOM70 is a protein that in humans is encoded by the TOMM70A gene.

Mitochondrial import inner membrane translocase subunit Tim23 is an enzyme that in humans is encoded by the TIMM23 gene.

Mitochondrial import inner membrane translocase subunit Tim17-A is an enzyme that in humans is encoded by the TIMM17A gene.

Translocase of outer mitochondrial membrane 40 homolog (yeast), also known as TOMM40, is a protein which in humans is encoded by the TOMM40 gene.

Mitochondrial import inner membrane translocase subunit Tim9 is an enzyme that in humans is encoded by the TIMM9 gene.

Mitochondrial import inner membrane translocase subunit TIM50 is an protein that in humans is encoded by the TIMM50 gene. Tim50 is a subunit of the Tim23 translocase complex in the inner mitochondrial membrane. Mutations in TIMM50 can lead to epilepsy, severe intellectual disability, and 3-methylglutaconic aciduria. TIMM50 expression is increased in breast cancer cells and decreased in hypertrophic hearts.
The translocase of the inner membrane (TIM) is a complex of proteins found in the inner mitochondrial membrane of the mitochondria. Components of the TIM complex facilitate the translocation of proteins across the inner membrane and into the mitochondrial matrix. They also facilitate the insertion of proteins into the inner mitochondrial membrane, where they must reside in order to function, these mainly include members of the mitochondrial carrier family of proteins.
Tim9 and Tim10 make up the group of essential small Tim proteins that assist in transport of hydrophobic precursors across the intermembrane space. Both Tim9 and Tim10 form a hexamer, the Tim9-Tim10 complex, that when associated, functions as a chaperone to assist translocation of preproteins from the outer mitochondrial membrane to the translocase of the inner membrane. The functional Tim9-Tim10 complex not only directs preproteins to the inner mitochondrial membrane in order to interact with the TIM22 complex, but also guides β-barrel precursor proteins to the sorting and assembly machinery (SAM) of the outer membrane.
Mitochondrial processing peptidase is an enzyme complex found in mitochondria which cleaves signal sequences from mitochondrial proteins. In humans this complex is composed of two subunits encoded by the genes PMPCA, and PMPCB. The enzyme is also known as. This enzyme catalyses the following chemical reaction
BamA is a β-barrel, outer membrane protein found in Gram-negative bacteria and it is the main and vital component of the β-barrel assembly machinery (BAM) complex in those bacteria. BAM Complex consists of five components; BamB, BamC, BamD, BamE and BamA. This complex is responsible in catalyzing folding and insertion of β-barrel proteins into the outer membrane of Gram-negative bacteria.