| Specific density | |
|---|---|
Common symbols | |
| SI unit | dimensionless |
Derivations from other quantities | |
| Specific density | |
|---|---|
Common symbols | |
| SI unit | dimensionless |
Derivations from other quantities | |
Specific density is a now obsolete term for relative density, which is the now preferred term. [1] [2]
Specific density is the density of an object divided by the density of a reference material. Specific gravity, a still-used but deprecated term, is the same as relative density. As an example, if a material has a specific density or relative density or specific gravity of 5, that means it has five times the density of some reference material (which should be named as part of the definition).
Specific density, being the same as relative density, is a dimensionless quantity. Usually specific density is density relative to water, but other types of relative density are possible (such as density relative to the density of dry air). [3] [4]
Density is the substance's mass per unit of volume. The symbol most often used for density is ρ, although the Latin letter D can also be used. Mathematically, density is defined as mass divided by volume:

Relative density, or specific gravity, is the ratio of the density of a substance to the density of a given reference material. Specific gravity for liquids is nearly always measured with respect to water at its densest ; for gases, the reference is air at room temperature. The term "relative density" is often preferred in scientific usage, whereas the term "specific gravity" is deprecated.

Gemology or gemmology is the science dealing with natural and artificial gemstone materials. It is a geoscience and a branch of mineralogy. Some jewelers are academically trained gemologists and are qualified to identify and evaluate gems.

The orbital period is the amount of time a given astronomical object takes to complete one orbit around another object. In astronomy, it usually applies to planets or asteroids orbiting the Sun, moons orbiting planets, exoplanets orbiting other stars, or binary stars. It may also refer to the time it takes a satellite orbiting a planet or moon to complete one orbit.
A hydrometer or lactometer is an instrument used for measuring density or relative density of liquids based on the concept of buoyancy. They are typically calibrated and graduated with one or more scales such as specific gravity.
Galilean invariance or Galilean relativity states that the laws of motion are the same in all inertial frames of reference. Galileo Galilei first described this principle in 1632 in his Dialogue Concerning the Two Chief World Systems using the example of a ship travelling at constant velocity, without rocking, on a smooth sea; any observer below the deck would not be able to tell whether the ship was moving or stationary.

Alcohol by volume is a standard measure of how much alcohol (ethanol) is contained in a given volume of an alcoholic beverage. It is defined as the number of millilitres (mL) of pure ethanol present in 100 mL of solution at 20 °C (68 °F). The number of millilitres of pure ethanol is the mass of the ethanol divided by its density at 20 °C (68 °F), which is 0.78945 g/ml. The ABV standard is used worldwide. The International Organization of Legal Metrology has tables of density of water–ethanol mixtures at different concentrations and temperatures.
The specific weight, also known as the unit weight, is the weight per unit volume of a material.
Degrees Brix is a measure of the dissolved solids in a liquid, and is commonly used to measure dissolved sugar content of an aqueous solution. One degree Brix is 1 gram of sucrose in 100 grams of solution and represents the strength of the solution as percentage by mass. If the solution contains dissolved solids other than pure sucrose, then the °Bx only approximates the dissolved solid content. For example, when one adds equal amounts of salt and sugar to equal amounts of water, the degrees of refraction (BRIX) of the salt solution rises faster than the sugar solution. The °Bx is traditionally used in the wine, sugar, carbonated beverage, fruit juice, fresh produce, maple syrup and honey industries.
The American Petroleum Institute gravity, or API gravity, is a measure of how heavy or light a petroleum liquid is compared to water: if its API gravity is greater than 10, it is lighter and floats on water; if less than 10, it is heavier and sinks.

In the field of extractive metallurgy, mineral processing is the process of separating commercially valuable minerals from their ores. Depending on the processes used in each instance, it is often also known as ore dressing or ore milling.
Specific energy or massic energy is energy per unit mass. It is also sometimes called gravimetric energy density, which is not to be confused with energy density, which is defined as energy per unit volume. It is used to quantify, for example, stored heat and other thermodynamic properties of substances such as specific internal energy, specific enthalpy, specific Gibbs free energy, and specific Helmholtz free energy. It may also be used for the kinetic energy or potential energy of a body. Specific energy is an intensive property, whereas energy and mass are extensive properties.
In thermodynamics, the specific volume of a substance is an intrinsic property of the substance, defined as the ratio of the substance's volume to its mass. It is the reciprocal of density ρ (rho) and it is related to the molar volume and molar mass:
The Baumé scale is a pair of hydrometer scales developed by French pharmacist Antoine Baumé in 1768 to measure density of various liquids. The unit of the Baumé scale has been notated variously as degrees Baumé, B°, Bé° and simply Baumé. One scale measures the density of liquids heavier than water and the other, liquids lighter than water. The Baumé of distilled water is 0. The API gravity scale is based on errors in early implementations of the Baumé scale.
Level sensors detect the level of liquids and other fluids and fluidized solids, including slurries, granular materials, and powders that exhibit an upper free surface. Substances that flow become essentially horizontal in their containers because of gravity whereas most bulk solids pile at an angle of repose to a peak. The substance to be measured can be inside a container or can be in its natural form. The level measurement can be either continuous or point values. Continuous level sensors measure level within a specified range and determine the exact amount of substance in a certain place, while point-level sensors only indicate whether the substance is above or below the sensing point. Generally the latter detect levels that are excessively high or low.

Gravity, in the context of fermenting alcoholic beverages, refers to the specific gravity, or relative density compared to water, of the wort or must at various stages in the fermentation. The concept is used in the brewing and wine-making industries. Specific gravity is measured by a hydrometer, refractometer, pycnometer or oscillating U-tube electronic meter.
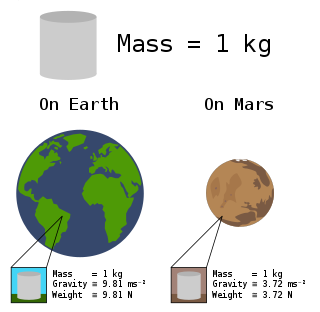
In common usage, the mass of an object is often referred to as its weight, though these are in fact different concepts and quantities. Nevertheless, one object will always weigh more than another with less mass if both are subject to the same gravity.
In brewing, attenuation refers to the conversion of sugars into alcohol and carbon dioxide by the fermentation process; the greater the attenuation, the more sugar has been converted into alcohol. A more attenuated beer is drier and more alcoholic than a less attenuated beer made from the same wort.
Jig concentrators are devices used mainly in the mining industry for mineral processing, to separate particles within the ore body, based on their specific gravity.
In the natural sciences, including physiology and engineering, a specific quantity generally refers to an intensive quantity obtained by dividing an extensive quantity of interest by mass. For example, specific leaf area is leaf area divided by leaf mass.