
Angiography or arteriography is a medical imaging technique used to visualize the inside, or lumen, of blood vessels and organs of the body, with particular interest in the arteries, veins, and the heart chambers. Modern angiography is performed by injecting a radio-opaque contrast agent into the blood vessel and imaging using X-ray based techniques such as fluoroscopy.

Optical coherence tomography (OCT) is an imaging technique that uses interferometry with short-coherence-length light to obtain micrometer-level depth resolution and uses transverse scanning of the light beam to form two- and three-dimensional images from light reflected from within biological tissue or other scattering media. Short-coherence-length light can be obtained using a superluminescent diode (SLD) with a broad spectral bandwidth or a broadly tunable laser with narrow linewidth. The first demonstration of OCT imaging was published by a team from MIT and Harvard Medical School in a 1991 article in the journal Science. The article introduced the term “OCT” to credit its derivation from optical coherence-domain reflectometry, in which the axial resolution is based on temporal coherence. The first demonstrations of in vivo OCT imaging quickly followed.
Medical optical imaging is the use of light as an investigational imaging technique for medical applications, pioneered by American Physical Chemist Britton Chance. Examples include optical microscopy, spectroscopy, endoscopy, scanning laser ophthalmoscopy, laser Doppler imaging, and optical coherence tomography. Because light is an electromagnetic wave, similar phenomena occur in X-rays, microwaves, and radio waves.

Functional near-infrared spectroscopy (fNIRS) is an optical brain monitoring technique which uses near-infrared spectroscopy for the purpose of functional neuroimaging. Using fNIRS, brain activity is measured by using near-infrared light to estimate cortical hemodynamic activity which occur in response to neural activity. Alongside EEG, fNIRS is one of the most common non-invasive neuroimaging techniques which can be used in portable contexts. The signal is often compared with the BOLD signal measured by fMRI and is capable of measuring changes both in oxy- and deoxyhemoglobin concentration, but can only measure from regions near the cortical surface. fNIRS may also be referred to as Optical Topography (OT) and is sometimes referred to simply as NIRS.

Optical tomography is a form of computed tomography that creates a digital volumetric model of an object by reconstructing images made from light transmitted and scattered through an object. Optical tomography is used mostly in medical imaging research. Optical tomography in industry is used as a sensor of thickness and internal structure of semiconductors.

Bruce J. Tromberg is an American photochemist and a leading researcher in the field of biophotonics. He is the director of the National Institute of Biomedical Imaging and Bioengineering (NIBIB) within the National Institutes of Health (NIH). Before joining NIH, he was Professor of Biomedical Engineering at The Henry Samueli School of Engineering and of Surgery at the School of Medicine, University of California, Irvine. He was the principal investigator of the Laser Microbeam and Medical Program (LAMMP), and the Director of the Beckman Laser Institute and Medical Clinic at Irvine. He was a co-leader of the Onco-imaging and Biotechnology Program of the NCI Chao Family Comprehensive Cancer Center at Irvine.
Microangiography is a type of angiography that consists of the radiography of small blood or lymphatic vessels of an organ. While most other types of angiography cannot produce images of vessels smaller than 200 µm in diameter, microangiography does just that. A microangiographic image is the result of injection of a contrast medium into either the blood or the lymphatic system and, then, enlargement of the resulting radiograph. Thus, an image is obtained in which there is contrast between vessel and surrounding tissue. It is often used in order to detect microvascular lesions in organs. But, it has been suggested that microangiography can also be used to detect tumors through visualization of tumor-induced small blood vessels. This is because tumor growths require vascularization before they can develop more rapidly. A few of the commonly used types are fluorescent, silicone rubber, and synchrotron radiation microangiography.
Ultrasound-modulated optical tomography (UOT), also known as Acousto-Optic Tomography (AOT), is a hybrid imaging modality that combines light and sound; it is a form of tomography involving ultrasound. It is used in imaging of biological soft tissues and has potential applications for early cancer detection. As a hybrid modality which uses both light and sound, UOT provides some of the best features of both: the use of light provides strong contrast and sensitivity ; these two features are derived from the optical component of UOT. The use of ultrasound allows for high resolution, as well as a high imaging depth. However, the difficulty of tackling the two fundamental problems with UOT have caused UOT to evolve relatively slowly; most work in the field is limited to theoretical simulations or phantom / sample studies.
Novacam Technologies Inc. specializes in designing and manufacturing advanced metrology and imaging systems for industrial and bio-medical applications. Novacam's fiber-based optical profilometers and Optical Coherence Tomography (OCT) systems are based on low coherence interferometry. The fiber-based nature of Novacam's detector probes is unique in the optical metrology industry.
James G. Fujimoto is Elihu Thomson Professor of Electrical Engineering and Computer Science at the Massachusetts Institute of Technology (MIT) and a visiting professor of ophthalmology at Tufts University School of Medicine, Boston, Massachusetts.
Lihong V. Wang is the Bren Professor of Medical Engineering and Electrical Engineering at the Andrew and Peggy Cherng Department of Medical Engineering at California Institute of Technology and was formerly the Gene K. Beare Distinguished Professorship of Biomedical Engineering at Washington University in St. Louis. Wang is renowned for his contributions to the field of Photoacoustic imaging technologies and inventing the world's fastest camera with more than 10 trillion frames per second. Wang was elected as the member of National Academy of Engineering (NAE) in 2018.

Intracoronary optical coherence tomography (OCT) is a catheter-based imaging application of optical coherence tomography. Currently prospective trials demonstrate OCT alters morbidity and/or mortality in coronary stenting and cervical cancer screening as discussed below.

Intravascular fluorescence is a catheter-based molecular imaging technique that uses near-infrared fluorescence to detect artery wall autofluorescence (NIRAF) or fluorescence generated by molecular agents injected intravenously (NIRF). No commercial systems based on intravascular fluorescence are currently on the market, however, significant steps forwards in intravascular fluorescence imaging technology have been made between 2010-2016. It is typically used to detect functional state of artery wall including some known high-risk features of atherosclerosis. It is usually combined with structural imaging modalities such as Intravascular ultrasound and/or Intracoronary optical coherence tomography, to provide functional information in a morphological context.
Super-resolution photoacoustic imaging is a set of techniques used to enhance spatial resolution in photoacoustic imaging. Specifically, these techniques primarily break the optical diffraction limit of the photoacoustic imaging system. It can be achieved in a variety of mechanisms, such as blind structured illumination, multi-speckle illumination, or photo-imprint photoacoustic microscopy in Figure 1.
Optical coherence elastography (OCE) is an emerging imaging technique used in biomedical imaging to form pictures of biological tissue in micron and submicron level and maps the biomechanical property of tissue.
Optical coherence tomography angiography (OCTA) is a non-invasive imaging technique based on optical coherence tomography (OCT) developed to visualize vascular networks in the human retina, choroid, skin and various animal models. OCTA may make use of speckle variance optical coherence tomography.
Time-domain diffuse optics or time-resolved functional near-infrared spectroscopy is a branch of functional near-Infrared spectroscopy which deals with light propagation in diffusive media. There are three main approaches to diffuse optics namely continuous wave (CW), frequency domain (FD) and time-domain (TD). Biological tissue in the range of red to near-infrared wavelengths are transparent to light and can be used to probe deep layers of the tissue thus enabling various in vivo applications and clinical trials.
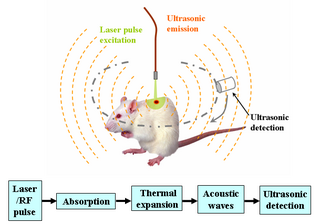
Deep learning in photoacoustic imaging combines the hybrid imaging modality of photoacoustic imaging (PA) with the rapidly evolving field of deep learning. Photoacoustic imaging is based on the photoacoustic effect, in which optical absorption causes a rise in temperature, which causes a subsequent rise in pressure via thermo-elastic expansion. This pressure rise propagates through the tissue and is sensed via ultrasonic transducers. Due to the proportionality between the optical absorption, the rise in temperature, and the rise in pressure, the ultrasound pressure wave signal can be used to quantify the original optical energy deposition within the tissue.

Christine P. Hendon is an electrical engineer and computer scientist and an associate professor in the Department of Electrical Engineering at Columbia University in New York City. Hendon is a pioneer in medical imaging. She develops biomedical optics technologies, using optical coherence tomography and near infrared spectroscopy systems, that enable physicians to perform guided interventional procedures and allow for structure-function dissection of human tissues and organs. Her advances in imaging technologies have led to improved diagnostic abilities and treatments for cardiac arrhythmias as well as breast cancer and preterm birth. She has been recognized for her development of optical imaging catheters for cardiac wall imaging by Forbes 30 under 30, the MIT Technology Review’s 35 Innovators Under 35, and by President Obama with the Presidential Early Career Awards in 2017.
Laser speckle contrast imaging (LSCI) which can also be called laser speckle imaging (LSI) is an imaging modality based on the analysis of the blurring effect of the speckle pattern. The operation of LSCI is having a wide-field illumination of a rough surface through a coherent light source. Then using photodetectors such as CCD camera or CMOS sensors imaging the resulting laser speckle pattern caused by the interference of coherent light. In biomedical use, the coherent light is typically in the red or near-infrared region to ensure higher penetration depth. When scattering particles moving during the time, the interference caused by the coherent light will have fluctuations which will lead to the intensity variations detected via the photodetector, and this change of the intensity contain the information of scattering particles' motion. Through image the speckle patterns with finite exposure time, areas with scattering particles will appear blurred.














