Related Research Articles
The gene rpoS encodes the sigma factor sigma-38, a 37.8 kD protein in Escherichia coli. Sigma factors are proteins that regulate transcription in bacteria. Sigma factors can be activated in response to different environmental conditions. rpoS is transcribed in late exponential phase, and RpoS is the primary regulator of stationary phase genes. RpoS is a central regulator of the general stress response and operates in both a retroactive and a proactive manner: it not only allows the cell to survive environmental challenges, but it also prepares the cell for subsequent stresses (cross-protection). The transcriptional regulator CsgD is central to biofilm formation, controlling the expression of the curli structural and export proteins, and the diguanylate cyclase, adrA, which indirectly activates cellulose production. The rpoS gene most likely originated in the gammaproteobacteria.

The 245 nucleotide sRNA of Escherichia coli, CsrC, was discovered using a genetic screen for factors that regulate glycogen biosynthesis. CsrC RNA binds multiple copies of CsrA, a protein that post-transcriptionally regulates central carbon flux, biofilm formation and motility in E. coli. CsrC antagonises the regulatory effects of CsrA, presumably by sequestering this protein. The discovery of CsrC is intriguing, in that a similar sRNA, CsrB, performs essentially the same function. Both sRNAs possess similar imperfect repeat sequences, primarily localised in the loops of predicted hairpins, which may serve as CsrA binding elements. Transcription of csrC increases as the culture approaches the stationary phase of growth and is indirectly activated by CsrA via the response regulator UvrY [1]. This RNA was also discovered in E. coli during a large scale screen [2]. The gene called SraK, was highly abundant in stationary phase, but low levels could be detected in exponentially growing cells as well [2].

The gcvB RNA gene encodes a small non-coding RNA involved in the regulation of a number of amino acid transport systems as well as amino acid biosynthetic genes. The GcvB gene is found in enteric bacteria such as Escherichia coli. GcvB regulates genes by acting as an antisense binding partner of the mRNAs for each regulated gene. This binding is dependent on binding to a protein called Hfq. Transcription of the GcvB RNA is activated by the adjacent GcvA gene and repressed by the GcvR gene. A deletion of GcvB RNA from Y. pestis changed colony shape as well as reducing growth. It has been shown by gene deletion that GcvB is a regulator of acid resistance in E. coli. GcvB enhances the ability of the bacterium to survive low pH by upregulating the levels of the alternate sigma factor RpoS. A polymeric form of GcvB has recently been identified. Interaction of GcvB with small RNA SroC triggers the degradation of GcvB by RNase E, lifting the GcvB-mediated mRNA repression of its target genes.

The SraC/RyeA RNA is a non-coding RNA that was discovered in E. coli during two large scale screens for RNAs. The function of this RNA is currently unknown. This RNA overlaps the SdsR/RyeB RNA on the opposite strand suggesting that the two RNAs may act in a concerted manner.

The OmrA-B RNA gene family is a pair of homologous OmpR-regulated small non-coding RNA that was discovered in E. coli during two large-scale screens. OmrA-B is highly abundant in stationary phase, but low levels could be detected in exponentially growing cells as well. RygB is adjacent to RygA a closely related RNA. These RNAs bind to the Hfq protein and regulate gene expression by antisense binding. They negatively regulate the expression of several genes encoding outer membrane proteins, including cirA, CsgD, fecA, fepA and ompT by binding in the vicinity of the Shine-Dalgarno sequence, suggesting the control of these targets is dependent on Hfq protein and RNase E. Taken together, these data suggest that OmrA-B participates in the regulation of outer membrane composition, responding to environmental conditions.
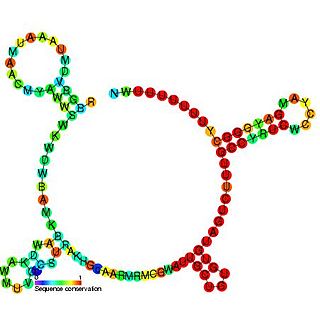
The RprA RNA gene encodes a 106 nucleotide regulatory non-coding RNA. Translational regulation of the stationary phase sigma factor RpoS is mediated by the formation of a double-stranded RNA stem-loop structure in the upstream region of the rpoS messenger RNA, occluding the translation initiation site.
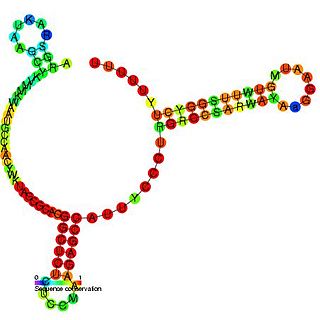
The SdsR/RyeB RNA is a non-coding RNA that was identified in a large scale screen of E. coli. The exact 5′ and 3′ ends of this RNA are uncertain. This RNA overlaps the SraC/RyeA RNA on the opposite strand suggesting that the two may act in a concerted manner. It is transcribed by general stress factor σs and is most highly expressed in stationary phase. SdsR/RyeB RNA interacts with Hfq.

RyhB RNA is a 90 nucleotide RNA that down-regulates a set of iron-storage and iron-using proteins when iron is limiting; it is itself negatively regulated by the ferric uptake repressor protein, Fur.

The SraB RNA is a small non-coding RNA discovered in E. coli during a large scale experimental screen. The 14 novel RNAs discovered were named 'sra' for small RNA, examples include SraC, SraD and SraG. This ncRNA was found to be expressed only in stationary phase. The exact function of this RNA is unknown but it has been shown to affect survival of Salmonella enterica to antibiotic administration in egg albumin. The authors suggest this may be due to SraB regulating a response to components in albumin.

The MicA RNA is a small non-coding RNA that was discovered in E. coli during a large scale screen. Expression of SraD is highly abundant in stationary phase, but low levels could be detected in exponentially growing cells as well.

SraG is a small non-coding RNA (ncRNA). It is the functional product of a gene which is not translated into protein.

In molecular biology the ArcZ RNA is a small non-coding RNA (ncRNA). It is the functional product of a gene which is not translated into protein. ArcZ is an Hfq binding RNA that functions as an antisense regulator of a number of protein coding genes.

GlmZ is a small non-coding RNA (ncRNA). It is the functional product of a gene which is not translated into protein.
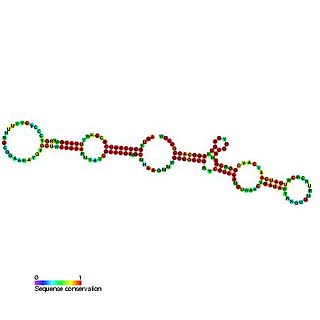
The GlmY RNA family consists of a number of bacterial RNA genes of around 167 bases in length. The GlmY RNA gene is present in Escherichia coli, Shigella flexneri, Yersinia pestis and Salmonella species, where it is found between the yfhK and purL genes. It was originally predicted in a bioinformatic screen for novel ncRNAs in E. coli.
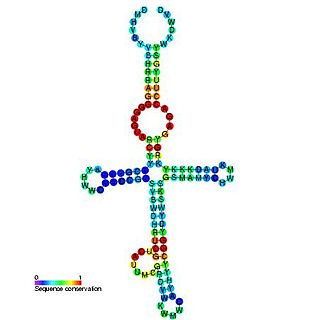
The yybP-ykoY leader RNA element was originally discovered in E. coli during a large scale screen and was named SraF. This family was later found to exist upstream of related families of protein genes in many bacteria, including the yybP and ykoY genes in B. subtilis. The specific functions of these proteins are unknown, but this structured RNA element may be involved in their genetic regulation as a riboswitch. The yybP-ykoY element was later proposed to be manganese-responsive after another associated family of genes, YebN/MntP, was shown to encode Mn2+ efflux pumps in several bacteria. Genetic data and a crystal structure confirmed that yybp-ykoY is a manganese riboswitch that directly binds Mn2+

The Hfq protein encoded by the hfq gene was discovered in 1968 as an Escherichia coli host factor that was essential for replication of the bacteriophage Qβ. It is now clear that Hfq is an abundant bacterial RNA binding protein which has many important physiological roles that are usually mediated by interacting with Hfq binding sRNA.

An Hfq binding sRNA is an sRNA that binds the bacterial RNA binding protein called Hfq. A number of bacterial small RNAs which have been shown to bind to Hfq have been characterised . Many of these RNAs share a similar structure composed of three stem-loops. Several studies have expanded this list, and experimentally validated a total of 64 Hfq binding sRNA in Salmonella Typhimurium. A transcriptome wide study on Hfq binding sites in Salmonella mapped 126 Hfq binding sites within sRNAs. Genomic SELEX has been used to show that Hfq binding RNAs are enriched in the sequence motif 5′-AAYAAYAA-3′. Genome-wide study identified 40 candidate Hfq-dependent sRNAs in plant pathogen Erwinia amylovora. 12 of them were confirmed by Northern blot.

FourU thermometers are a class of non-coding RNA thermometers found in Salmonella. They are named 'FourU' due to the four highly conserved uridine nucleotides found directly opposite the Shine-Dalgarno sequence on hairpin II (pictured). RNA thermometers such as FourU control regulation of temperature via heat shock proteins in many prokaryotes. FourU thermometers are relatively small RNA molecules, only 57 nucleotides in length, and have a simple two-hairpin structure.
Bacterial small RNAs (sRNA) are small RNAs produced by bacteria; they are 50- to 500-nucleotide non-coding RNA molecules, highly structured and containing several stem-loops. Numerous sRNAs have been identified using both computational analysis and laboratory-based techniques such as Northern blotting, microarrays and RNA-Seq in a number of bacterial species including Escherichia coli, the model pathogen Salmonella, the nitrogen-fixing alphaproteobacterium Sinorhizobium meliloti, marine cyanobacteria, Francisella tularensis, Streptococcus pyogenes, the pathogen Staphylococcus aureus, and the plant pathogen Xanthomonas oryzae pathovar oryzae. Bacterial sRNAs affect how genes are expressed within bacterial cells via interaction with mRNA or protein, and thus can affect a variety of bacterial functions like metabolism, virulence, environmental stress response, and structure.
Escherichia coli contains a number of small RNAs located in intergenic regions of its genome. The presence of at least 55 of these has been verified experimentally. 275 potential sRNA-encoding loci were identified computationally using the QRNA program. These loci will include false positives, so the number of sRNA genes in E. coli is likely to be less than 275. A computational screen based on promoter sequences recognised by the sigma factor sigma 70 and on Rho-independent terminators predicted 24 putative sRNA genes, 14 of these were verified experimentally by northern blotting. The experimentally verified sRNAs included the well characterised sRNAs RprA and RyhB. Many of the sRNAs identified in this screen, including RprA, RyhB, SraB and SraL, are only expressed in the stationary phase of bacterial cell growth. A screen for sRNA genes based on homology to Salmonella and Klebsiella identified 59 candidate sRNA genes. From this set of candidate genes, microarray analysis and northern blotting confirmed the existence of 17 previously undescribed sRNAs, many of which bind to the chaperone protein Hfq and regulate the translation of RpoS. UptR sRNA transcribed from the uptR gene is implicated in suppressing extracytoplasmic toxicity by reducing the amount of membrane-bound toxic hybrid protein.
References
- 1 2 Argaman, L.; Hershberg, R.; Vogel, J.; Bejerano, G.; Wagner, E. G.; Margalit, H.; Altuvia, S. (2001-06-26). "Novel small RNA-encoding genes in the intergenic regions of Escherichia coli". Current Biology. 11 (12): 941–950. doi:10.1016/s0960-9822(01)00270-6. ISSN 0960-9822. PMID 11448770.
- ↑ Wassarman, K. M.; Repoila, F.; Rosenow, C.; Storz, G.; Gottesman, S. (2001-07-01). "Identification of novel small RNAs using comparative genomics and microarrays". Genes & Development. 15 (13): 1637–1651. doi:10.1101/gad.901001. ISSN 0890-9369. PMC 312727 . PMID 11445539.
- 1 2 Viegas, Sandra C.; Pfeiffer, Verena; Sittka, Alexandra; Silva, Inês J.; Vogel, Jörg; Arraiano, Cecília M. (2007). "Characterization of the role of ribonucleases in Salmonella small RNA decay". Nucleic Acids Research. 35 (22): 7651–7664. doi:10.1093/nar/gkm916. ISSN 1362-4962. PMC 2190706 . PMID 17982174.
- ↑ Ortega, Alvaro D.; Gonzalo-Asensio, Jesús; García-del Portillo, Francisco (April 2012). "Dynamics of Salmonella small RNA expression in non-growing bacteria located inside eukaryotic cells". RNA Biology. 9 (4): 469–488. doi: 10.4161/rna.19317 . ISSN 1555-8584. PMID 22336761.
- ↑ Silva, Inês Jesus; Ortega, Alvaro Darío; Viegas, Sandra Cristina; García-Del Portillo, Francisco; Arraiano, Cecília Maria (September 2013). "An RpoS-dependent sRNA regulates the expression of a chaperone involved in protein folding". RNA. 19 (9): 1253–1265. doi:10.1261/rna.039537.113. ISSN 1469-9001. PMC 3753932 . PMID 23893734.
| This molecular or cell biology article is a stub. You can help Wikipedia by expanding it. |