
Atomic absorption spectroscopy (AAS) and atomic emission spectroscopy (AES) is a spectroanalytical procedure for the quantitative determination of chemical elements using the absorption of optical radiation (light) by free atoms in the gaseous state. Atomic absorption spectroscopy is based on absorption of light by free metallic ions.
Fourier-transform spectroscopy is a measurement technique whereby spectra are collected based on measurements of the coherence of a radiative source, using time-domain or space-domain measurements of the electromagnetic radiation or other type of radiation. It can be applied to a variety of types of spectroscopy including optical spectroscopy, infrared spectroscopy, nuclear magnetic resonance (NMR) and magnetic resonance spectroscopic imaging (MRSI), mass spectrometry and electron spin resonance spectroscopy. There are several methods for measuring the temporal coherence of the light, including the continuous wave Michelson or Fourier-transform spectrometer and the pulsed Fourier-transform spectrograph.
In solid-state physics, the work function is the minimum thermodynamic work needed to remove an electron from a solid to a point in the vacuum immediately outside the solid surface. Here "immediately" means that the final electron position is far from the surface on the atomic scale, but still too close to the solid to be influenced by ambient electric fields in the vacuum. The work function is not a characteristic of a bulk material, but rather a property of the surface of the material.

Raman spectroscopy ; named after Indian physicist Sir C. V. Raman) is a spectroscopic technique used to observe vibrational, rotational, and other low-frequency modes in a system. Raman spectroscopy is commonly used in chemistry to provide a structural fingerprint by which molecules can be identified.

Ultraviolet–visible spectroscopy or ultraviolet–visible spectrophotometry refers to absorption spectroscopy or reflectance spectroscopy in the ultraviolet-visible spectral region. This means it uses light in the visible and adjacent ranges. The absorption or reflectance in the visible range directly affects the perceived color of the chemicals involved. In this region of the electromagnetic spectrum, atoms and molecules undergo electronic transitions. Absorption spectroscopy is complementary to fluorescence spectroscopy, in that fluorescence deals with transitions from the excited state to the ground state, while absorption measures transitions from the ground state to the excited state.
A spectral line is a dark or bright line in an otherwise uniform and continuous spectrum, resulting from emission or absorption of light in a narrow frequency range, compared with the nearby frequencies. Spectral lines are often used to identify atoms and molecules. These "fingerprints" can be compared to the previously collected "fingerprints" of atoms and molecules, and are thus used to identify the atomic and molecular components of stars and planets, which would otherwise be impossible.

Absorption spectroscopy refers to spectroscopic techniques that measure the absorption of radiation, as a function of frequency or wavelength, due to its interaction with a sample. The sample absorbs energy, i.e., photons, from the radiating field. The intensity of the absorption varies as a function of frequency, and this variation is the absorption spectrum. Absorption spectroscopy is performed across the electromagnetic spectrum.
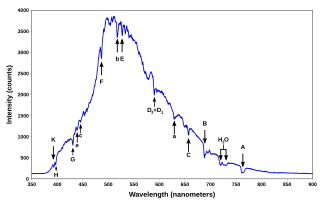
Plasma diagnostics are a pool of methods, instruments, and experimental techniques used to measure properties of a plasma, such as plasma components' density, distribution function over energy (temperature), their spatial profiles and dynamics, which enable to derive plasma parameters.

Fluorescence spectroscopy is a type of electromagnetic spectroscopy that analyzes fluorescence from a sample. It involves using a beam of light, usually ultraviolet light, that excites the electrons in molecules of certain compounds and causes them to emit light; typically, but not necessarily, visible light. A complementary technique is absorption spectroscopy. In the special case of single molecule fluorescence spectroscopy, intensity fluctuations from the emitted light are measured from either single fluorophores, or pairs of fluorophores.
In physics and physical chemistry, time-resolved spectroscopy is the study of dynamic processes in materials or chemical compounds by means of spectroscopic techniques. Most often, processes are studied after the illumination of a material occurs, but in principle, the technique can be applied to any process that leads to a change in properties of a material. With the help of pulsed lasers, it is possible to study processes that occur on time scales as short as 10−16 seconds.

In physics, terahertz time-domain spectroscopy (THz-TDS) is a spectroscopic technique in which the properties of matter are probed with short pulses of terahertz radiation. The generation and detection scheme is sensitive to the sample's effect on both the amplitude and the phase of the terahertz radiation. By measuring in the time-domain, the technique can provide more information than conventional Fourier-transform spectroscopy, which is only sensitive to the amplitude. Since the time-domain, and consequently the frequency-domain, of the THz signal is available, the distorting effect of the diffraction can be mitigated and the resolution of the THz images can be enhanced substantially. This resolution enhancement process is illustrated in the Figure to the right.
Resonance Raman spectroscopy is a Raman spectroscopy technique in which the incident photon energy is close in energy to an electronic transition of a compound or material under examination. The frequency coincidence can lead to greatly enhanced intensity of the Raman scattering, which facilitates the study of chemical compounds present at low concentrations.

Mössbauer spectroscopy is a spectroscopic technique based on the Mössbauer effect. This effect, discovered by Rudolf Mössbauer in 1958, consists in the nearly recoil-free, resonant absorption and emission of gamma rays in solids.
Chemical imaging is the analytical capability to create a visual image of components distribution from simultaneous measurement of spectra and spatial, time information. Hyperspectral imaging measures contiguous spectral bands, as opposed to multispectral imaging which measures spaced spectral bands.
Photothermal spectroscopy is a group of high sensitivity spectroscopy techniques used to measure optical absorption and thermal characteristics of a sample. The basis of photothermal spectroscopy is the change in thermal state of the sample resulting from the absorption of radiation. Light absorbed and not lost by emission results in heating. The heat raises temperature thereby influencing the thermodynamic properties of the sample or of a suitable material adjacent to it. Measurement of the temperature, pressure, or density changes that occur due to optical absorption are ultimately the basis for the photothermal spectroscopic measurements.
The quantum-confined Stark effect (QCSE) describes the effect of an external electric field upon the light absorption spectrum or emission spectrum of a quantum well (QW). In the absence of an external electric field, electrons and holes within the quantum well may only occupy states within a discrete set of energy subbands. Only a discrete set of frequencies of light may be absorbed or emitted by the system. When an external electric field is applied, the electron states shift to lower energies, while the hole states shift to higher energies. This reduces the permitted light absorption or emission frequencies. Additionally, the external electric field shifts electrons and holes to opposite sides of the well, decreasing the overlap integral, which in turn reduces the recombination efficiency of the system. The spatial separation between the electrons and holes is limited by the presence of the potential barriers around the quantum well, meaning that excitons are able to exist in the system even under the influence of an electric field. The quantum-confined Stark effect is used in QCSE optical modulators, which allow optical communications signals to be switched on and off rapidly.
Photoelectrochemical processes are processes in photoelectrochemistry; they usually involve transforming light into other forms of energy. These processes apply to photochemistry, optically pumped lasers, sensitized solar cells, luminescence, and photochromism.

Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong static magnetic field are perturbed by a weak oscillating magnetic field and respond by producing an electromagnetic signal with a frequency characteristic of the magnetic field at the nucleus. This process occurs near resonance, when the oscillation frequency matches the intrinsic frequency of the nuclei, which depends on the strength of the static magnetic field, the chemical environment, and the magnetic properties of the isotope involved; in practical applications with static magnetic fields up to ca. 20 tesla, the frequency is similar to VHF and UHF television broadcasts (60–1000 MHz). NMR results from specific magnetic properties of certain atomic nuclei. Nuclear magnetic resonance spectroscopy is widely used to determine the structure of organic molecules in solution and study molecular physics, crystals as well as non-crystalline materials. NMR is also routinely used in advanced medical imaging techniques, such as in magnetic resonance imaging (MRI).
Terahertz spectroscopy detects and controls properties of matter with electromagnetic fields that are in the frequency range between a few hundred gigahertz and several terahertz. In many-body systems, several of the relevant states have an energy difference that matches with the energy of a THz photon. Therefore, THz spectroscopy provides a particularly powerful method in resolving and controlling individual transitions between different many-body states. By doing this, one gains new insights about many-body quantum kinetics and how that can be utilized in developing new technologies that are optimized up to the elementary quantum level.












