This page is based on this
Wikipedia article Text is available under the
CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.

Optical brighteners, optical brightening agents (OBAs), fluorescent brightening agents (FBAs), or fluorescent whitening agents (FWAs), are chemical compounds that absorb light in the ultraviolet and violet region of the electromagnetic spectrum, and re-emit light in the blue region by fluorescence. Fluorescent emission is a short-lived period of light emission by a fluorophore, unlike phosphorescence, which is long-lived. These additives are often used to enhance the appearance of color of fabric and paper, causing a "whitening" effect; they make intrinsically yellow/orange materials look less so, by compensating the deficit in blue and purple light reflected by the material, with the blue and purple optical emission of the fluorophore.

(E)-Stilbene, commonly known as trans-stilbene, is an organic compound represented by the condensed structural formula C6H5CH=CHC6H5. Classified as a diarylethene, it features a central ethylene moiety with one phenyl group substituents on each end of the carbon–carbon double bond. It has an (E) stereochemistry, meaning that the phenyl groups are located on opposite sides of the double bond, the opposite of its geometric isomer, cis-stilbene. Trans-stilbene occurs as a white crystalline solid at room temperature and is highly soluble in organic solvents. It can be converted to cis-stilbene photochemically, and further reacted to produce phenanthrene.

Stilbenoids are hydroxylated derivatives of stilbene. They have a C6–C2–C6 structure. In biochemical terms, they belong to the family of phenylpropanoids and share most of their biosynthesis pathway with chalcones. Stilbenoids can be produced by plants and bacteria.
In enzymology, a phloroisovalerophenone synthase (EC 2.3.1.156) is an enzyme that catalyzes the chemical reaction

(Z)-Stilbene is a diarylethene, that is, a hydrocarbon consisting of a cis ethene double bond substituted with a phenyl group on both carbon atoms of the double bond. The name stilbene was derived from the Greek word stilbos, which means shining.
Stilbene may refer to one of the two stereoisomers of 1,2-diphenylethene:

α-Viniferin is a stilbene trimer. It can be isolated from Caragana chamlagu and from Caragana sinica and from the stem bark of Dryobalanops aromatica. It is also present in relation to resistance to Botrytis cinerea and Plasmopara viticola in Vitis vinifera and Vitis riparia.
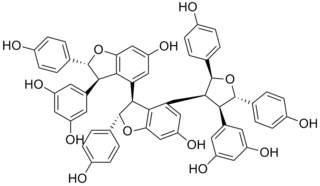
Kobophenol A is a stilbenoid. It is a tetramer of resveratrol. It can be isolated from Caragana chamlagu, from Caragana sinica and from Carex folliculata seeds.
Coumaroyl-coenzyme A is a chemical compound found in plants. The compound is the thioester of coenzyme-A and coumaric acid.

Olivetol, also known as 5-pentylresorcinol or 5-pentyl-1,3-benzenediol, is an organic compound found in certain species of lichen; it is also a precursor in various syntheses of tetrahydrocannabinol.
The biosynthesis of phenylpropanoids involves a number of enzymes.
Trans-resveratrol di-O-methyltransferase is an enzyme with systematic name S-adenosyl-L-methionine:trans-resveratrol 3,5-O-dimethyltransferase. This enzyme catalyses the following chemical reaction
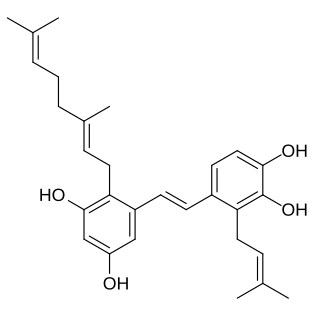
Pawhuskin A is a naturally occurring prenylated stilbene isolated from Dalea purpurea which acts as a competitive silent antagonist of the κ-, μ-, and δ-opioid receptors. The compound was named after Pawhuska, Oklahoma, a place near where the samples of Dalea purpurea that led to its discovery were taken from. Other isolates of the plant with affinity for opioid receptors include pawhuskin B and pawhuskin C, though these compounds produce comparatively weak opioid receptor displacement relative to pawhuskin A. Dalea purpurea was used in traditional Native American medicine to treat various ailments, and pawhuskin A and related isolates may be some of the constituents of the plant which underlay this use.

Ketoacyl synthases (KSs) catalyze the condensation reaction of acyl-CoA or acyl-acyl ACP with malonyl-CoA to form 3-ketoacyl-CoA or with malonyl-ACP to form 3-ketoacyl-ACP. This reaction is a key step in the fatty acid synthesis cycle, as the resulting acyl chain is two carbon atoms longer than before. KSs exist as individual enzymes, as they do in type II fatty acid synthesis and type II polyketide synthesis, or as domains in large multidomain enzymes, such as type I fatty acid synthases (FASs) and polyketide synthases (PKSs). KSs are divided into five families: KS1, KS2, KS3, KS4, and KS5.








