
Meiosis is a special type of cell division of germ cells and apicomplexans in sexually-reproducing organisms that produces the gametes, the sperm or egg cells. It involves two rounds of division that ultimately result in four cells, each with only one copy of each chromosome (haploid). Additionally, prior to the division, genetic material from the paternal and maternal copies of each chromosome is crossed over, creating new combinations of code on each chromosome. Later on, during fertilisation, the haploid cells produced by meiosis from a male and a female will fuse to create a zygote, a cell with two copies of each chromosome again.

Gametogenesis is a biological process by which diploid or haploid precursor cells undergo cell division and differentiation to form mature haploid gametes. Depending on the biological life cycle of the organism, gametogenesis occurs by meiotic division of diploid gametocytes into various gametes, or by mitosis. For example, plants produce gametes through mitosis in gametophytes. The gametophytes grow from haploid spores after sporic meiosis. The existence of a multicellular, haploid phase in the life cycle between meiosis and gametogenesis is also referred to as alternation of generations.

A germ cell is any cell that gives rise to the gametes of an organism that reproduces sexually. In many animals, the germ cells originate in the primitive streak and migrate via the gut of an embryo to the developing gonads. There, they undergo meiosis, followed by cellular differentiation into mature gametes, either eggs or sperm. Unlike animals, plants do not have germ cells designated in early development. Instead, germ cells can arise from somatic cells in the adult, such as the floral meristem of flowering plants.

Spermatogenesis is the process by which haploid spermatozoa develop from germ cells in the seminiferous tubules of the testicle. This process starts with the mitotic division of the stem cells located close to the basement membrane of the tubules. These cells are called spermatogonial stem cells. The mitotic division of these produces two types of cells. Type A cells replenish the stem cells, and type B cells differentiate into primary spermatocytes. The primary spermatocyte divides meiotically into two secondary spermatocytes; each secondary spermatocyte divides into two equal haploid spermatids by Meiosis II. The spermatids are transformed into spermatozoa (sperm) by the process of spermiogenesis. These develop into mature spermatozoa, also known as sperm cells. Thus, the primary spermatocyte gives rise to two cells, the secondary spermatocytes, and the two secondary spermatocytes by their subdivision produce four spermatozoa and four haploid cells.

Oogenesis, ovogenesis, or oögenesis is the differentiation of the ovum into a cell competent to further develop when fertilized. It is developed from the primary oocyte by maturation. Oogenesis is initiated in the embryonic stage.
David C. Page is an American biologist and professor at the Massachusetts Institute of Technology (MIT), the director of the Whitehead Institute, and a Howard Hughes Medical Institute (HHMI) investigator. He is best known for his work on mapping the Y-chromosome and on its evolution in mammals and expression during development. He was cited by Bryan Sykes in Adam's Curse: A Future Without Men.

Spermatocytes are a type of male gametocyte in animals. They derive from immature germ cells called spermatogonia. They are found in the testis, in a structure known as the seminiferous tubules. There are two types of spermatocytes, primary and secondary spermatocytes. Primary and secondary spermatocytes are formed through the process of spermatocytogenesis.
Gametogonium are stem cells for gametes located within the gonads. They originate from primordial germ cells, which have migrated to the gonads. Male gametogonia which are located within the testes during development and adulthood are called spermatogonium. Female gametogonia, known as oogonium, are found within the ovaries of the developing foetus and were thought to be depleted at or after birth. Spermatogonia and oogonia are classified as sexually differentiated germ cells.

Retinoic acid (used simplified here for all-trans-retinoic acid) is a metabolite of vitamin A1 (all-trans-retinol) that mediates the functions of vitamin A1 required for growth and development. All-trans-retinoic acid is required in chordate animals, which includes all higher animals from fish to humans. During early embryonic development, all-trans-retinoic acid generated in a specific region of the embryo helps determine position along the embryonic anterior/posterior axis by serving as an intercellular signaling molecule that guides development of the posterior portion of the embryo. It acts through Hox genes, which ultimately control anterior/posterior patterning in early developmental stages.
In developmental biology, the cells that give rise to the gametes are often set aside during embryonic cleavage. During development, these cells will differentiate into primordial germ cells, migrate to the location of the gonad, and form the germline of the animal.
cAMP responsive element modulator is a protein that in humans is encoded by the CREM gene, and it belongs to the cAMP-responsive element binding protein family. It has multiple isoforms, which act either as repressors or activators. CREB family is important for in regulating transcription in response to various stresses, metabolic and developmental signals. CREM transcription factors also play an important role in many physiological systems, such as cardiac function, circadian rhythms, locomotion and spermatogenesis.

Deleted in azoospermia 1, also known as DAZ1, is a protein which in humans is encoded by the DAZ1 gene.

Exonuclease 1 is an enzyme that in humans is encoded by the EXO1 gene.

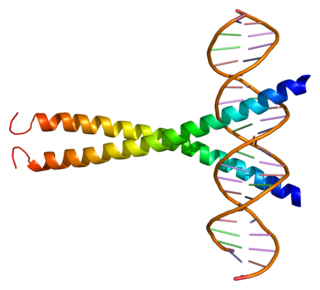
Synaptonemal complex protein 3 is a protein that in humans is encoded by the SYCP3 gene. It is a component of the synaptonemal complex formed between homologous chromosomes during the prophase of meiosis.

Serine/threonine-protein kinase MAK is an enzyme that in humans is encoded by the MAK gene.

Cytochrome P450 26B1 is a protein that in humans is encoded by the CYP26B1 gene.

The meiotic recombination checkpoint monitors meiotic recombination during meiosis, and blocks the entry into metaphase I if recombination is not efficiently processed.

Folliculogenesis-specific basic helix-loop-helix, also known as factor in the germline alpha (FIGalpha) or transcription factor FIGa, is a protein that in humans is encoded by the FIGLA gene. The FIGLA gene is a germ cell-specific transcription factor preferentially expressed in oocytes that can be found on human chromosome 2p13.3.

A spermatogonial stem cell (SSC), also known as a type A spermatogonium, is a spermatogonium that does not differentiate into a spermatocyte, a precursor of sperm cells. Instead, they continue dividing into other spermatogonia or remain dormant to maintain a reserve of spermatogonia. Type B spermatogonia, on the other hand, differentiate into spermatocytes, which in turn undergo meiosis to eventually form mature sperm cells.
The DAZprotein family is a group of three highly conserved RNA-binding proteins that are important in gametogenesis and meiosis. Therefore, mutations in the genes that encode for the DAZ proteins can have detrimental consequences for fertility.















