Strontium ruthenate may refer to two compounds:
- Monostrontium ruthenate, SrRuO3, a ferromagnetic perovskite.
- Distrontium ruthenate, Sr2RuO4, a perovskite superconductor that does not contain copper.
Strontium ruthenate may refer to two compounds:

Ruthenium is a chemical element with the symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most other chemicals. Russian-born scientist of Baltic-German ancestry Karl Ernst Claus discovered the element in 1844 at Kazan State University and named ruthenium in honor of Russia. Ruthenium is usually found as a minor component of platinum ores; the annual production has risen from about 19 tonnes in 2009 to some 35.5 tonnes in 2017. Most ruthenium produced is used in wear-resistant electrical contacts and thick-film resistors. A minor application for ruthenium is in platinum alloys and as a chemistry catalyst. A new application of ruthenium is as the capping layer for extreme ultraviolet photomasks. Ruthenium is generally found in ores with the other platinum group metals in the Ural Mountains and in North and South America. Small but commercially important quantities are also found in pentlandite extracted from Sudbury, Ontario, and in pyroxenite deposits in South Africa.
In physical chemistry and engineering, passivation is coating a material so that it becomes "passive", that is, less readily affected or corroded by the environment. Passivation involves creation of an outer layer of shield material that is applied as a microcoating, created by chemical reaction with the base material, or allowed to build by spontaneous oxidation in the air. As a technique, passivation is the use of a light coat of a protective material, such as metal oxide, to create a shield against corrosion. Passivation of silicon is used during fabrication of microelectronic devices. Undesired passivation of electrodes, called "fouling", increases the circuit resistance so it interferes with some electrochemical applications such as electrocoagulation for wastewater treatment, amperometric chemical sensing, and electrochemical synthesis.

A perovskite is any material with a crystal structure following the formula ABX3, which was first discovered as the mineral called perovskite, which consists of calcium titanium oxide (CaTiO3). The mineral was first discovered in the Ural mountains of Russia by Gustav Rose in 1839 and named after Russian mineralogist L. A. Perovski (1792–1856). 'A' and 'B' are two positively charged ions (i.e. cations), often of very different sizes, and X is a negatively charged ion (an anion, frequently oxide) that bonds to both cations. The 'A' atoms are generally larger than the 'B' atoms. The ideal cubic structure has the B cation in 6-fold coordination, surrounded by an octahedron of anions, and the A cation in 12-fold cuboctahedral coordination. Additional perovskite forms may exist where either/both the A and B sites have a configuration of A1x-1A2x and/or B1y-1B2y and the X may deviate from the ideal coordination configuration as ions within the A and B sites undergo changes in their oxidation states.
PPV, ppv or pPv may refer to:

Strontium titanate is an oxide of strontium and titanium with the chemical formula SrTiO3. At room temperature, it is a centrosymmetric paraelectric material with a perovskite structure. At low temperatures it approaches a ferroelectric phase transition with a very large dielectric constant ~104 but remains paraelectric down to the lowest temperatures measured as a result of quantum fluctuations, making it a quantum paraelectric. It was long thought to be a wholly artificial material, until 1982 when its natural counterpart—discovered in Siberia and named tausonite—was recognised by the IMA. Tausonite remains an extremely rare mineral in nature, occurring as very tiny crystals. Its most important application has been in its synthesized form wherein it is occasionally encountered as a diamond simulant, in precision optics, in varistors, and in advanced ceramics.
PV may refer to:

The core–mantle boundary (CMB) of Earth lies between the planet's silicate mantle and its liquid iron–nickel outer core, at a depth of 2,891 km (1,796 mi) below Earth's surface. The boundary is observed via the discontinuity in seismic wave velocities at that depth due to the differences between the acoustic impedances of the solid mantle and the molten outer core. P-wave velocities are much slower in the outer core than in the deep mantle while S-waves do not exist at all in the liquid portion of the core. Recent evidence suggests a distinct boundary layer directly above the CMB possibly made of a novel phase of the basic perovskite mineralogy of the deep mantle named post-perovskite. Seismic tomography studies have shown significant irregularities within the boundary zone and appear to be dominated by the African and Pacific Large Low-Shear-Velocity Provinces (LLSVP).

Perovskite (pronunciation: ) is a calcium titanium oxide mineral composed of calcium titanate (chemical formula CaTiO3). Its name is also applied to the class of compounds which have the same type of crystal structure as CaTiO3, known as the perovskite structure, which has a general chemical formula A2+B4+(X2−)3. Many different cations can be embedded in this structure, allowing the development of diverse engineered materials.
LSAT may refer to:

Lead(II) thiocyanate is a compound, more precisely a salt, with the formula Pb(SCN)2. It is a white crystalline solid, but will turn yellow upon exposure to light. It is slightly soluble in water and can be converted to a basic salt (Pb(CNS)2·Pb(OH)2 when boiled. Salt crystals may form upon cooling. Lead thiocyanate can cause lead poisoning if ingested and can adversely react with many substances. It has use in small explosives, matches, and dyeing.

Calcium titanate is an inorganic compound with the chemical formula Ca Ti O3. As a mineral, it is called perovskite, named after Russian mineralogist, L. A. Perovski (1792-1856). It is a colourless, diamagnetic solid, although the mineral is often coloured owing to impurities.
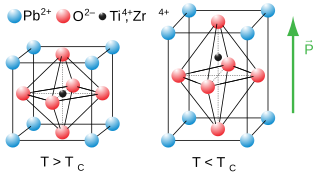
Lead(II) titanate is an inorganic compound with the chemical formula PbTiO3. It is the lead salt of titanic acid. Lead(II) titanate is a yellow powder that is insoluble in water.

Distrontium ruthenate, also known as strontium ruthenate, is an oxide of strontium and ruthenium with the chemical formula Sr2RuO4. It was the first reported perovskite superconductor that did not contain copper. Strontium ruthenate is structurally very similar to the high-temperature cuprate superconductors, and in particular, is almost identical to the lanthanum doped superconductor (La, Sr)2CuO4. However, the transition temperature for the superconducting phase transition is 0.93 K (about 1.5 K for the best sample), which is much lower than the corresponding value for cuprates.
Goldschmidt's tolerance factor (from the German word Toleranzfaktor) is an indicator for the stability and distortion of crystal structures. It was originally only used to describe the perovskite ABO3 structure, but now tolerance factors are also used for ilmenite.
Silicate perovskite is either (Mg,Fe)SiO3 or CaSiO3 when arranged in a perovskite structure. Silicate perovskites are not stable at Earth's surface, and mainly exist in the lower part of Earth's mantle, between about 670 and 2,700 km depth. They are thought to form the main mineral phases, together with ferropericlase.
Lanthanum manganite is an inorganic compound with the formula LaMnO3, often abbreviated as LMO. Lanthanum manganite is formed in the perovskite structure, consisting of oxygen octahedra with a central Mn atom. The cubic perovskite structure is distorted into an orthorhombic structure by a strong Jahn–Teller distortion of the oxygen octahedra.

A perovskite solar cell (PSC) is a type of solar cell that includes a perovskite-structured compound, most commonly a hybrid organic–inorganic lead or tin halide-based material as the light-harvesting active layer. Perovskite materials, such as methylammonium lead halides and all-inorganic cesium lead halide, are cheap to produce and simple to manufacture.

Henry James Snaith is a professor in physics in the Clarendon Laboratory at the University of Oxford. Research from his group has led to the creation of a new research field, based on halide perovskites for use as solar absorbers. Many individuals who were PhD students and postdoctoral researchers in Snaith's group have now established research groups, independent research portfolios and commercial enterprises. He co-founded Oxford Photovoltaics in 2010 to commercialise perovskite based tandem solar cells.
Monostrontium ruthenate is the inorganic compound with the formula SrRuO3. It is one of two main strontium ruthenates, the other having the formula Sr2RuO4. SrRuO3 is a ferromagnetic. It has a perovskite structure as do many complex metal oxides with the ABO3 formula. The Ru4+ ions occupy the octahedral sites and the larger Sr2+ ions are distorted 12-coordinate.
Barium ruthenate is an inorganic compound, with the chemical formula of BaRuO3. It can be obtained from the stoichiometric reaction of barium oxide and ruthenium(IV) oxide at temperatures below 1200 °C, or from the thermal decomposition of Ba[Ru(NO)(NO2)4(OH)]·xH2O. It reacts with ruthenium and ruthenium(IV) oxide at 1250 °C to obtain black needle-like crystal BaRu6O12. Hydrogen or zirconium can reduce it when heated to produce metal ruthenium.