
An electronic portfolio is a collection of electronic evidence assembled and managed by a user, usually but not only on the Web.

Current good manufacturing practices (cGMP) are those conforming to the guidelines recommended by relevant agencies. Those agencies control the authorization and licensing of the manufacture and sale of food and beverages, cosmetics, pharmaceutical products, dietary supplements, and medical devices. These guidelines provide minimum requirements that a manufacturer must meet to assure that their products are consistently high in quality, from batch to batch, for their intended use.
Electronic assessment, also known as digital assessment, e-assessment, online assessment or computer-based assessment, is the use of information technology in assessment such as educational assessment, health assessment, psychiatric assessment, and psychological assessment. This covers a wide range of activities ranging from the use of a word processor for assignments to on-screen testing. Specific types of e-assessment include multiple choice, online/electronic submission, computerized adaptive testing such as the Frankfurt Adaptive Concentration Test, and computerized classification testing.
A subscription-based product of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), MedDRA or Medical Dictionary for Regulatory Activities is a clinically validated international medical terminology dictionary-thesaurus used by regulatory authorities and the biopharmaceutical industry during the regulatory process, from pre-marketing to post-marketing activities, and for safety information data entry, retrieval, evaluation, and presentation. Also, it is the adverse event classification dictionary.
Pharmacovigilance, also known as drug safety, is the pharmaceutical science relating to the "collection, detection, assessment, monitoring, and prevention" of adverse effects with pharmaceutical products.
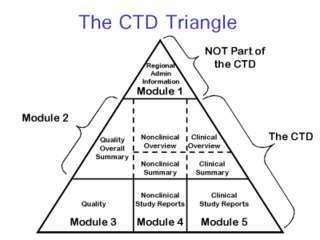
The Common Technical Document (CTD) is a set of specifications for an application dossier for the registration of medicine, designed for use across Europe, Japan, the United States, and beyond.
Medical practice management software (PMS) is a category of healthcare software that deals with the day-to-day operations of a medical practice including veterinarians. Such software frequently allows users to capture patient demographics, schedule appointments, maintain lists of insurance payors, perform billing tasks, and generate reports.
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) is an initiative that brings together regulatory authorities and pharmaceutical industry to discuss scientific and technical aspects of pharmaceutical product development and registration. The mission of the ICH is to promote public health by achieving greater harmonisation through the development of technical guidelines and requirements for pharmaceutical product registration.
Enterprise content management (ECM) extends the concept of content management by adding a timeline for each content item and, possibly, enforcing processes for its creation, approval, and distribution. Systems using ECM generally provide a secure repository for managed items, analog or digital. They also include one methods for importing content to manage new items, and several presentation methods to make items available for use. Although ECM content may be protected by digital rights management (DRM), it is not required. ECM is distinguished from general content management by its cognizance of the processes and procedures of the enterprise for which it is created.

An academic conference or scientific conference is an event for researchers to present and discuss their scholarly work. Together with academic or scientific journals and preprint archives, conferences provide an important channel for exchange of information between researchers. Further benefits of participating in academic conferences include learning effects in terms of presentation skills and "academic habitus", receiving feedback from peers for one's own research, the possibility to engage in informal communication with peers about work opportunities and collaborations, and getting an overview of current research in one or more disciplines.
Abstract management is the process of accepting and preparing abstracts for presentation at an academic conference. The process consists of either invited or proffered submissions of the abstract or summary of work. The abstract typically states the hypothesis, tools used in research or investigation, data collected, and a summary or interpretation of the data.
The electronic common technical document (eCTD) is an interface and international specification for the pharmaceutical industry to agency transfer of regulatory information. The specification is based on the Common Technical Document (CTD) format and was developed by the International Council for Harmonisation (ICH) Multidisciplinary Group 2 Expert Working Group.
An ideas bank is a widely available shared resource, usually a website, where people post, exchange, discuss, and polish new ideas. Some ideas banks are used to develop new inventions or technologies. Many corporations have installed internal ideas banks to gather the input from their employees and improve their ideation process. Some ideas banks employ a voting system to estimate an idea's value. In some cases, ideas banks can be more humor-oriented than their serious counterparts. The underlying theory of an ideas bank is that if a large group of people collaborate on a project or the development of an idea that eventually said project or idea will reach perfection in the eyes of those who worked on it.
The following outline is provided as an overview of and topical guide to clinical research:
Regulated Product Submission (RPS) is a Health Level Seven (HL7) standard designed to facilitate the processing and review of regulated product information. RPS is being developed in response to performance goals that the U.S. Food and Drug Administration (FDA) is to achieve by 2012, as outlined in the Prescription Drug User Fee Act (PDUFA). In addition to the U.S., regulatory agencies from Europe, Canada, and Japan are at varying levels of interest and participation. Currently, the second release of RPS is in development.
Electronic submission refers to the submission of a document by electronic means: that is, via e-mail or a web form on the Internet, or on an electronic medium such as a compact disc, a hard disk or a USB flash drive. Traditionally, the term "manuscript" referred to anything that was explicitly "written by hand". However, in popular usage and especially in the context of computers and the internet, the term "manuscript" may even refer to documents typed out or prepared on typewriters and computers and can be extended to digital photographs and videos, and online surveys too. In other words, any manuscript prepared and submitted online can be considered to be an electronic submission.
A submission management system is a software system, also known as submission processing, that streamlines and eases out the collection, tracking and management of electronic submissions. Information can be received, authenticated, tracked, stored, and distributed electronically. Submission management systems can be web-based system operating in a browser environment, a COTS based product, or may also be in the form of a desktop application. Submissions are completed electronically creating an efficient real-time process that saves time for both the submitter and recipient. Usually a submission management system can take in a high volume of data at fast rate.
In order to comply with government regulatory requirements pertinent to clinical trials, every organization involved in clinical trials must maintain and store certain documents, images and content related to the clinical trial. Depending on the regulatory jurisdiction, this information may be stored in the trial master file or TMF, which today takes the form of an electronic trial master file (eTMF). The International Conference on Harmonization (ICH) published a consolidated guidance for industry on Good Clinical Practice in 1996 with the objective of providing a unified standard for the European Union, Japan, and the United States of America to facilitate mutual acceptance of clinical data by the regulatory authorities in those jurisdictions. This guidance document established the requirement across all ICH regions to establish trial master files containing essential documents that individually and collectively permit evaluation of the conduct of a trial and the quality of the data produced.[2] In some jurisdictions, for example the USA, there is no specific requirement for a trial master file. However, if the regulatory authority requires ICH GCP to be followed, then there is consequently a requirement to create and maintain a trial master file.[2]
Diary studies is a research method that collects qualitative information by having participants record entries about their everyday lives in a log, diary or journal about the activity or experience being studied. This collection of data uses a longitudinal technique, meaning participants are studied over a period of time. This research tool, although not being able to provide results as detailed as a true field study, can still offer a vast amount of contextual information without the costs of a true field study. Diary studies are also known as experience sampling or ecological momentary assessment (EMA) methodology.
Grievance Redressal is a management- and governance-related process used commonly in India. While the term "Grievance Redressal" primarily covers the receipt and processing of complaints from citizens and consumers, a wider definition includes actions taken on any issue raised by them to avail services more effectively.



