Related Research Articles

Molybdopterins are a class of cofactors found in most molybdenum-containing and all tungsten-containing enzymes. Synonyms for molybdopterin are: MPT and pyranopterin-dithiolate. The nomenclature for this biomolecule can be confusing: Molybdopterin per se contains no molybdenum; rather, this is the name of the ligand that will bind the active metal. After molybdopterin is eventually complexed with molybdenum, the complete ligand is usually called molybdenum cofactor.

Sulfite oxidase is an enzyme in the mitochondria of all eukaryotes, with exception of the yeasts. It oxidizes sulfite to sulfate and, via cytochrome c, transfers the electrons produced to the electron transport chain, allowing generation of ATP in oxidative phosphorylation. This is the last step in the metabolism of sulfur-containing compounds and the sulfate is excreted.

Sulfur assimilation is the process by which organisms obtain sulfur, an essential element for growth and metabolism of most organisms. Biologically, sulfur is often encountered in its most oxidized form as sulfate or the most reduced form as hydrogen sulfide or organosulfur compounds, such as the two amino acids cysteine and methionine.
Microbial metabolism is the means by which a microbe obtains the energy and nutrients it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other based on metabolic characteristics. The specific metabolic properties of a microbe are the major factors in determining that microbe's ecological niche, and often allow for that microbe to be useful in industrial processes or responsible for biogeochemical cycles.

Nitrate reductases are molybdoenzymes that reduce nitrate to nitrite. This reaction is critical for the production of protein in most crop plants, as nitrate is the predominant source of nitrogen in fertilized soils.
Sulfite reductase (ferredoxin) (EC 1.8.7.1, ferredoxin-sulfite reductase) is an enzyme with systematic name hydrogen-sulfide:ferredoxin oxidoreductase. This enzyme catalises the following chemical reaction

In enzymology, a sulfite dehydrogenase (EC 1.8.2.1) is an enzyme that catalyzes the chemical reaction
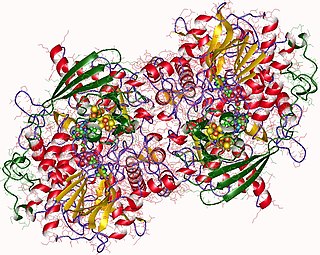
Adenylyl-sulfate reductase is an enzyme that catalyzes the chemical reaction of the reduction of adenylyl-sulfate/adenosine-5'-phosphosulfate (APS) to sulfite through the use of an electron donor cofactor. The products of the reaction are AMP and sulfite, as well as an oxidized electron donor cofactor.
Adenylyl-sulfate reductase (glutathione) is an enzyme that catalyzes the chemical reaction
Adenylyl-sulfate reductase (thioredoxin) is an enzyme that catalyzes the chemical reaction
In enzymology, a ferredoxin—nitrite reductase (EC 1.7.7.1) is an enzyme that catalyzes the chemical reaction
In enzymology, a hydrogensulfite reductase (EC 1.8.99.3) is an enzyme that catalyzes the chemical reaction
In enzymology, a phosphoadenylyl-sulfate reductase (thioredoxin) is an enzyme that catalyzes the chemical reaction

Sulfite reductases (EC 1.8.99.1) are enzymes that participate in sulfur metabolism. They catalyze the reduction of sulfite to hydrogen sulfide and water. Electrons for the reaction are provided by a dissociable molecule of either NADPH, bound flavins, or ferredoxins.
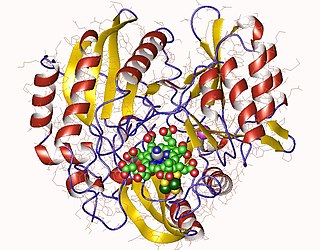
Sulfite reductase (NADPH) (EC 1.8.1.2, sulfite (reduced nicotinamide adenine dinucleotide phosphate) reductase, NADPH-sulfite reductase, NADPH-dependent sulfite reductase, H2S-NADP oxidoreductase, sulfite reductase (NADPH2)) is an enzyme with systematic name hydrogen-sulfide:NADP+ oxidoreductase. This enzyme catalises the following chemical reaction
Sulfur is metabolized by all organisms, from bacteria and archaea to plants and animals. Sulfur is reduced or oxidized by organisms in a variety of forms. The element is present in proteins, sulfate esters of polysaccharides, steroids, phenols, and sulfur-containing coenzymes.
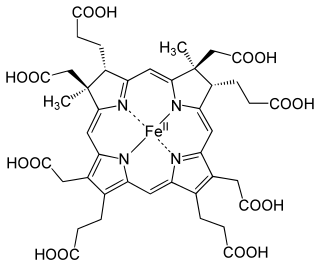
Siroheme is a heme-like prosthetic group at the active sites of some enzymes to accomplish the six-electron reduction of sulfur and nitrogen. It is a cofactor at the active site of sulfite reductase, which plays a major role in sulfur assimilation pathway, converting sulfite into sulfide, which can be incorporated into the organic compound homocysteine.

Dissimilatory sulfate reduction is a form of anaerobic respiration that uses sulfate as the terminal electron acceptor to produce hydrogen sulfide. This metabolism is found in some types of bacteria and archaea which are often termed sulfate-reducing organisms. The term "dissimilatory" is used when hydrogen sulfide is produced in an anaerobic respiration process. By contrast, the term "assimilatory" would be used in relation to the biosynthesis of organosulfur compounds, even though hydrogen sulfide may be an intermediate.
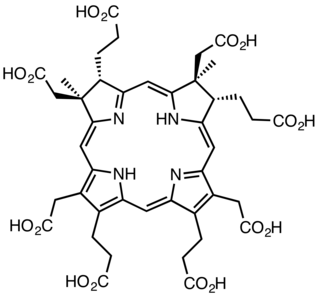
Sirohydrochlorin is a tetrapyrrole macrocyclic metabolic intermediate in the biosynthesis of sirohaem, the iron-containing prosthetic group in sulfite reductase enzymes. It is also the biosynthetic precursor to cofactor F430, an enzyme which catalyzes the release of methane in the final step of methanogenesis.
Dissimilatory sulfite reductase is an enzyme that participates in sulfur metabolism in dissimilatory sulfate reduction.