
Biopolymers are natural polymers produced by the cells of living organisms. Like other polymers, biopolymers consist of monomeric units that are covalently bonded in chains to form larger molecules. There are three main classes of biopolymers, classified according to the monomers used and the structure of the biopolymer formed: polynucleotides, polypeptides, and polysaccharides. The Polynucleotides, RNA and DNA, are long polymers of nucleotides. Polypeptides include proteins and shorter polymers of amino acids; some major examples include collagen, actin, and fibrin. Polysaccharides are linear or branched chains of sugar carbohydrates; examples include starch, cellulose, and alginate. Other examples of biopolymers include natural rubbers, suberin and lignin, cutin and cutan, melanin, and polyhydroxyalkanoates (PHAs).
Surface modification is the act of modifying the surface of a material by bringing physical, chemical or biological characteristics different from the ones originally found on the surface of a material. This modification is usually made to solid materials, but it is possible to find examples of the modification to the surface of specific liquids.

Biocompatibility is related to the behavior of biomaterials in various contexts. The term refers to the ability of a material to perform with an appropriate host response in a specific situation. The ambiguity of the term reflects the ongoing development of insights into how biomaterials interact with the human body and eventually how those interactions determine the clinical success of a medical device. Modern medical devices and prostheses are often made of more than one material so it might not always be sufficient to talk about the biocompatibility of a specific material. Even the same materials, such as diamond-like carbon coatings, may show different levels of biocompatibility based on the manufacturing conditions and characteristics.

Plasma cleaning is the removal of impurities and contaminants from surfaces through the use of an energetic plasma or dielectric barrier discharge (DBD) plasma created from gaseous species. Gases such as argon and oxygen, as well as mixtures such as air and hydrogen/nitrogen are used. The plasma is created by using high frequency voltages to ionise the low pressure gas, although atmospheric pressure plasmas are now also common.
A biomaterial is a substance that has been engineered to interact with biological systems for a medical purpose – either a therapeutic or a diagnostic one. The corresponding field of study, called biomaterials science or biomaterials engineering, is about fifty years old. It has experienced steady growth over its history, with many companies investing large amounts of money into the development of new products. Biomaterials science encompasses elements of medicine, biology, chemistry, tissue engineering and materials science.

A foreign body reaction (FBR) is a typical tissue response to a foreign body within biological tissue. It usually includes the formation of a foreign body granuloma. Tissue-encapsulation of an implant is an example, as is inflammation around a splinter. Foreign body granuloma formation consists of protein adsorption, macrophages, multinucleated foreign body giant cells, fibroblasts, and angiogenesis. It has also been proposed that the mechanical property of the interface between an implant and its surrounding tissues is critical for the host response.
Biomimetic materials are materials developed using inspiration from nature. This may be useful in the design of composite materials. Natural structures have inspired and innovated human creations. Notable examples of these natural structures include: honeycomb structure of the beehive, strength of spider silks, bird flight mechanics, and shark skin water repellency. The etymological roots of the neologism "biomimetic" derive from Greek, since bios means "life" and mimetikos means "imitative".
A nerve guidance conduit is an artificial means of guiding axonal regrowth to facilitate nerve regeneration and is one of several clinical treatments for nerve injuries. When direct suturing of the two stumps of a severed nerve cannot be accomplished without tension, the standard clinical treatment for peripheral nerve injuries is autologous nerve grafting. Due to the limited availability of donor tissue and functional recovery in autologous nerve grafting, neural tissue engineering research has focused on the development of bioartificial nerve guidance conduits as an alternative treatment, especially for large defects. Similar techniques are also being explored for nerve repair in the spinal cord but nerve regeneration in the central nervous system poses a greater challenge because its axons do not regenerate appreciably in their native environment.
Nitinol biocompatibility is an important factor in biomedical applications. Nitinol (NiTi), which is formed by alloying nickel and titanium, is a shape-memory alloy with superelastic properties more similar to that of bone, when compared to stainless steel, another commonly used biomaterial. Biomedical applications that utilize nitinol include stents, heart valve tools, bone anchors, staples, septal defect devices and implants. It is a commonly used biomaterial especially in the development of stent technology.
Nano-scaffolding or nanoscaffolding is a medical process used to regrow tissue and bone, including limbs and organs. The nano-scaffold is a three-dimensional structure composed of polymer fibers very small that are scaled from a Nanometer scale. Developed by the American military, the medical technology uses a microscopic apparatus made of fine polymer fibers called a scaffold. Damaged cells grip to the scaffold and begin to rebuild missing bone and tissue through tiny holes in the scaffold. As tissue grows, the scaffold is absorbed into the body and disappears completely.

Bioceramics and bioglasses are ceramic materials that are biocompatible. Bioceramics are an important subset of biomaterials. Bioceramics range in biocompatibility from the ceramic oxides, which are inert in the body, to the other extreme of resorbable materials, which are eventually replaced by the body after they have assisted repair. Bioceramics are used in many types of medical procedures. Bioceramics are typically used as rigid materials in surgical implants, though some bioceramics are flexible. The ceramic materials used are not the same as porcelain type ceramic materials. Rather, bioceramics are closely related to either the body's own materials or are extremely durable metal oxides.

Arginylglycylaspartic acid (RGD) is the most common peptide motif responsible for cell adhesion to the extracellular matrix (ECM), found in species ranging from Drosophila to humans. Cell adhesion proteins called integrins recognize and bind to this sequence, which is found within many matrix proteins, including fibronectin, fibrinogen, vitronectin, osteopontin, and several other adhesive extracellular matrix proteins. The discovery of RGD and elucidation of how RGD binds to integrins has led to the development of a number of drugs and diagnostics, while the peptide itself is used ubiquitously in bioengineering. Depending on the application and the integrin targeted, RGD can be chemically modified or replaced by a similar peptide which promotes cell adhesion.
Adsorption is the accumulation and adhesion of molecules, atoms, ions, or larger particles to a surface, but without surface penetration occurring. The adsorption of larger biomolecules such as proteins is of high physiological relevance, and as such they adsorb with different mechanisms than their molecular or atomic analogs. Some of the major driving forces behind protein adsorption include: surface energy, intermolecular forces, hydrophobicity, and ionic or electrostatic interaction. By knowing how these factors affect protein adsorption, they can then be manipulated by machining, alloying, and other engineering techniques to select for the most optimal performance in biomedical or physiological applications.
Polymeric materials have widespread application due to their versatile characteristics, cost-effectiveness, and highly tailored production. The science of polymer synthesis allows for excellent control over the properties of a bulk polymer sample. However, surface interactions of polymer substrates are an essential area of study in biotechnology, nanotechnology, and in all forms of coating applications. In these cases, the surface characteristics of the polymer and material, and the resulting forces between them largely determine its utility and reliability. In biomedical applications for example, the bodily response to foreign material, and thus biocompatibility, is governed by surface interactions. In addition, surface science is integral part of the formulation, manufacturing, and application of coatings.
An antimicrobial surface is coated by an antimicrobial agent that inhibits the ability of microorganisms to grow on the surface of a material. Such surfaces are becoming more widely investigated for possible use in various settings including clinics, industry, and even the home. The most common and most important use of antimicrobial coatings has been in the healthcare setting for sterilization of medical devices to prevent hospital associated infections, which have accounted for almost 100,000 deaths in the United States. In addition to medical devices, linens and clothing can provide a suitable environment for many bacteria, fungi, and viruses to grow when in contact with the human body which allows for the transmission of infectious disease.

Titanium was first introduced into surgeries in the 1950s after having been used in dentistry for a decade prior. It is now the metal of choice for prosthetics, internal fixation, inner body devices, and instrumentation. Titanium is used from head to toe in biomedical implants. One can find titanium in neurosurgery, bone conduction hearing aids, false eye implants, spinal fusion cages, pacemakers, toe implants, and shoulder/elbow/hip/knee replacements along with many more. The main reason why titanium is often used in the body is due to titanium's biocompatibility and, with surface modifications, bioactive surface. The surface characteristics that affect biocompatibility are surface texture, steric hindrance, binding sites, and hydrophobicity (wetting). These characteristics are optimized to create an ideal cellular response. Some medical implants, as well as parts of surgical instruments are coated with titanium nitride (TiN).
Biomaterials exhibit various degrees of compatibility with the harsh environment within a living organism. They need to be nonreactive chemically and physically with the body, as well as integrate when deposited into tissue. The extent of compatibility varies based on the application and material required. Often modifications to the surface of a biomaterial system are required to maximize performance. The surface can be modified in many ways, including plasma modification and applying coatings to the substrate. Surface modifications can be used to affect surface energy, adhesion, biocompatibility, chemical inertness, lubricity, sterility, asepsis, thrombogenicity, susceptibility to corrosion, degradation, and hydrophilicity.

Bovine submaxillary mucin (BSM) coatings are a surface treatment provided to biomaterials intended to reduce the growth of disadvantageous bacteria and fungi such as S. epidermidis, E. coli, and Candida albicans. BSM is a substance extracted from the fresh salivary glands of cows. It exhibits unique physical properties, such as high molecular weight and amphiphilicity, that allow it to be used for many biomedical applications.
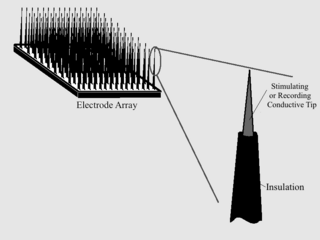
As with any material implanted in the body, it is important to minimize or eliminate foreign body response and maximize effectual integration. Neural implants have the potential to increase the quality of life for patients with such disabilities as Alzheimer's, Parkinson's, epilepsy, depression, and migraines. With the complexity of interfaces between a neural implant and brain tissue, adverse reactions such as fibrous tissue encapsulation that hinder the functionality, occur. Surface modifications to these implants can help improve the tissue-implant interface, increasing the lifetime and effectiveness of the implant.
A chronic electrode implant is an electronic device implanted chronically into the brain or other electrically excitable tissue. It may record electrical impulses in the brain or may stimulate neurons with electrical impulses from an external source.

















