Related Research Articles

The Controlled Substances Act (CSA) is the statute establishing federal U.S. drug policy under which the manufacture, importation, possession, use, and distribution of certain substances is regulated. It was passed by the 91st United States Congress as Title II of the Comprehensive Drug Abuse Prevention and Control Act of 1970 and signed into law by President Richard Nixon. The Act also served as the national implementing legislation for the Single Convention on Narcotic Drugs.

3,4-Methyl

The Drug Enforcement Administration is a United States federal law enforcement agency under the U.S. Department of Justice tasked with combating drug trafficking and distribution within the U.S. It is the lead agency for domestic enforcement of the Controlled Substances Act, sharing concurrent jurisdiction with the Federal Bureau of Investigation, the U.S. Immigration and Customs Enforcement, and U.S. Customs and Border Protection. The DEA has sole responsibility for coordinating and pursuing U.S. drug investigations both domestically and abroad.

Methaqualone is a sedative and hypnotic medication. It was sold under the brand names Quaalude and Sopor among others, which contained 300 mg of methaqualone, and sold as a combination drug under the brand name Mandrax, which contained 250 mg methaqualone and 25 mg diphenhydramine within the same tablet, mostly in Europe. Commercial production of methaqualone was halted in the mid-1980s due to widespread abuse and addictiveness. It is a member of the quinazolinone class.
Clandestine chemistry is chemistry carried out in secret, and particularly in illegal drug laboratories. Larger labs are usually run by gangs or organized crime intending to produce for distribution on the black market. Smaller labs can be run by individual chemists working clandestinely in order to synthesize smaller amounts of controlled substances or simply out of a hobbyist interest in chemistry, often because of the difficulty in ascertaining the purity of other, illegally synthesized drugs obtained on the black market. The term clandestine lab is generally used in any situation involving the production of illicit compounds, regardless of whether the facilities being used qualify as a true laboratory.

The United States Anti-Doping Agency is a non-profit, non-governmental 501(c)(3) organization and the national anti-doping organization (NADO) for the United States. To protect clean competition and the integrity of sport and prevent doping in the United States with a performance-enhancing substance, the USADA provides education, leads scientific initiatives, conducts testing, and oversees the results management process. Headquartered in Colorado Springs, Colorado, USADA is a signatory to the World Anti-Doping Code, which harmonizes anti-doping practices around the world and is widely considered the basis for the strongest and strictest anti-doping programs to prevent doping in sport.

In the United States, the removal of cannabis from Schedule I of the Controlled Substances Act, has been proposed repeatedly since 1972. The category is the most tightly restricted category reserved for drugs that have "no currently accepted medical use.”

The Comprehensive Drug Abuse Prevention and Control Act of 1970, Pub.L. 91–513, 84 Stat. 1236, enacted October 27, 1970, is a United States federal law that, with subsequent modifications, requires the pharmaceutical industry to maintain physical security and strict record keeping for certain types of drugs. Controlled substances are divided into five schedules on the basis of their potential for abuse, accepted medical use, and accepted safety under medical supervision. Substances in Schedule I have a high potential for abuse, no accredited medical use, and a lack of accepted safety. From Schedules II to V, substances decrease in potential for abuse. The schedule a substance is placed in determines how it must be controlled. Prescriptions for drugs in all schedules must bear the physician's federal Drug Enforcement Administration (DEA) license number, but some drugs in Schedule V do not require a prescription. State schedules may vary from federal schedules.
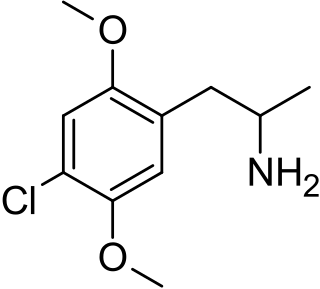
2,5-Dimethoxy-4-chloroamphetamine (DOC) is a psychedelic drug of the phenethylamine and amphetamine chemical classes. It was presumably first synthesized by Alexander Shulgin, and was described in his book PiHKAL.

Butorphanol is a morphinan-type synthetic agonist–antagonist opioid analgesic developed by Bristol-Myers. Butorphanol is most closely structurally related to levorphanol. Butorphanol is available as the tartrate salt in injectable, tablet, and intranasal spray formulations. The tablet form is only used in dogs, cats and horses due to low bioavailability in humans.

Hydrocodone/paracetamol is the combination of the pain medications hydrocodone and paracetamol (acetaminophen). It is used to treat moderate to severe pain. It is taken by mouth. Recreational use is common in the United States.

2C-H (2,5-dimethoxyphenethylamine) is a lesser-known substituted phenethylamine of the 2C family.

THC acetate ester is the acetate ester of THC.

The drug policy of the United States is the activity of the federal government relating to the regulation of drugs. Consumer drugs are regulated by the Food and Drug Administration (FDA) while the Drug Enforcement Administration (DEA) is tasked with enforcing laws against drug distribution.

Embutramide is a potent opioid analgesic and sedative drug that is structurally related to methadone. It was developed by Hoechst A.G. in 1958 and was investigated as a general anesthetic agent, but was found to have a very narrow therapeutic window, with a 50 mg/kg dose producing effective sedation and a 75 mg/kg dose being fatal. Along with strong sedative effects, embutramide also produces respiratory depression and ventricular arrhythmia. Because of these properties, it was never adopted for medical use as an anesthetic as it was considered too dangerous for this purpose. Instead it is used for euthanasia in veterinary medicine, mainly for the euthanization of dogs.
Drug precursors, also referred to as precursor chemicals or simply precursors, are substances which are known to be used in the illegal manufacture of illicit drugs. Most precursors also have legitimate commercial uses and are legally used in a wide variety of industrial processes and consumer products, such as medicines, flavourings, and fragrances.

Methamphetamine in the United States is regulated under Schedule II of the Controlled Substances Act. It is approved for pharmacological use in the treatment of attention deficit hyperactivity disorder and treatment-resistant obesity, but it is primarily used as a recreational drug. In 2012, 16,000 prescriptions for methamphetamine were filled, approximately 1.2 million Americans reported using it in the past year, and 440,000 reported using the drug in the past month.

The Designer Anabolic Steroid Control Act of 2014 is a bill that expanded the list of anabolic steroids regulated by the Drug Enforcement Administration (DEA) to include about two dozen new substances and established new crimes relating to false labeling of steroids. The bill established a penalty of up to $500,000 against those found to be falsely labeling their anabolic steroid products.

Mitragynine is an indole-based alkaloid and the most abundant active alkaloid in the Southeast Asian plant Mitragyna speciosa, commonly known as kratom. The total alkaloid concentration in dried leaves ranges from 0.5 to 1.5%. In Thai varieties, mitragynine is the most abundant component while 7-hydroxymitragynine is a minor constituent. In Malaysian kratom varieties, mitragynine is present at lower concentration. Such preparations are orally consumed and typically involve dried kratom leaves which are brewed into tea or ground and placed into capsules. Mitragynine consumption for medicinal and recreation purposes dates back centuries, although early use was primarily limited to Southeast Asian countries such as Indonesia and Thailand where the plant grows indigenously. Recently, mitragynine use has spread throughout Europe and the Americas as both a recreational and medicinal drug. While research into the effects of kratom have begun to emerge, investigations on the active compound mitragynine are less common.
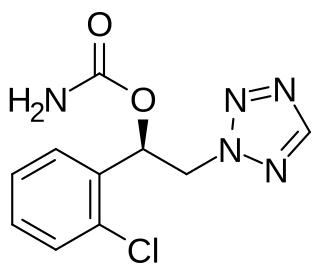
Cenobamate, sold under the brand names Xcopri (US) and Ontozry (EU), is a medication used for the treatment of partial-onset seizures, a kind of epilepsy, in adults. It is taken by mouth.
References
- 1 2 "System to Retrieve Information From Drug Evidence II", US-DEA
- ↑ "The Economic Impact of the Illicit Drug Industry", Transnational Institute, December 2003
- ↑ "Data for Monitoring the Nation's Drug Problems", Informing America's Policy on Illegal Drugs, 2001, doi:10.17226/10021, ISBN 978-0-309-07273-1
- ↑ "Bulletin on Narcotics" (PDF), UN Offices on Drugs and Crime, vol. 56, no. 1–2, 2004