
A rotaxane is a mechanically interlocked molecular architecture consisting of a dumbbell-shaped molecule which is threaded through a macrocycle. The two components of a rotaxane are kinetically trapped since the ends of the dumbbell are larger than the internal diameter of the ring and prevent dissociation (unthreading) of the components since this would require significant distortion of the covalent bonds.

Nanoid robotics, or for short, nanorobotics or nanobotics, is an emerging technology field creating machines or robots, which are called nanorobots or simply nanobots, whose components are at or near the scale of a nanometer. More specifically, nanorobotics refers to the nanotechnology engineering discipline of designing and building nanorobots with devices ranging in size from 0.1 to 10 micrometres and constructed of nanoscale or molecular components. The terms nanobot, nanoid, nanite, nanomachine and nanomite have also been used to describe such devices currently under research and development.

Supramolecular chemistry refers to the branch of chemistry concerning chemical systems composed of a discrete number of molecules. The strength of the forces responsible for spatial organization of the system range from weak intermolecular forces, electrostatic charge, or hydrogen bonding to strong covalent bonding, provided that the electronic coupling strength remains small relative to the energy parameters of the component. While traditional chemistry concentrates on the covalent bond, supramolecular chemistry examines the weaker and reversible non-covalent interactions between molecules. These forces include hydrogen bonding, metal coordination, hydrophobic forces, van der Waals forces, pi–pi interactions and electrostatic effects.

A nanomotor is a molecular or nanoscale device capable of converting energy into movement. It can typically generate forces on the order of piconewtons.
Artificial photosynthesis is a chemical process that biomimics the natural process of photosynthesis. The term artificial photosynthesis is used loosely, referring to any scheme for capturing and then storing energy from sunlight by producing a fuel, specifically a solar fuel. An advantage of artificial photosynthesis would be that the solar energy could converted and stored. By contrast, using photovoltaic cells, sunlight is converted into electricity and then converted again into chemical energy for storage, with some necessary losses of energy associated with the second conversion. The byproducts of these reactions are environmentally friendly. Artificially photosynthesized fuel would be a carbon-neutral source of energy, but it has never been demonstrated in any practical sense. The economics of artificial photosynthesis are noncompetitive.

Molecular machines are a class of molecules typically described as an assembly of a discrete number of molecular components intended to produce mechanical movements in response to specific stimuli, mimicking macromolecular devices such as switches and motors. Naturally occurring or biological molecular machines are responsible for vital living processes such as DNA replication and ATP synthesis. Kinesins and ribosomes are examples of molecular machines, and they often take the form of multi-protein complexes. For the last several decades, scientists have attempted, with varying degrees of success, to miniaturize machines found in the macroscopic world. The first example of an artificial molecular machine (AMM) was reported in 1994, featuring a rotaxane with a ring and two different possible binding sites. In 2016 the Nobel Prize in Chemistry was awarded to Jean-Pierre Sauvage, Sir J. Fraser Stoddart, and Bernard L. Feringa for the design and synthesis of molecular machines.

In host–guest chemistry, cucurbiturils are macrocyclic molecules made of glycoluril monomers linked by methylene bridges. The oxygen atoms are located along the edges of the band and are tilted inwards, forming a partly enclosed cavity (cavitand). The name is derived from the resemblance of this molecule with a pumpkin of the family of Cucurbitaceae.

Molecular tweezers, and molecular clips, are host molecules with open cavities capable of binding guest molecules. The open cavity of the molecular tweezers may bind guests using non-covalent bonding, which includes hydrogen bonding, metal coordination, hydrophobic forces, van der Waals forces, π–π interactions, and/or electrostatic effects. These complexes are a subset of macrocyclic molecular receptors and their structure is that the two "arms" that bind the guest molecule between them are only connected at one end leading to a certain flexibility of these receptor molecules.

Molecular motors are natural (biological) or artificial molecular machines that are the essential agents of movement in living organisms. In general terms, a motor is a device that consumes energy in one form and converts it into motion or mechanical work; for example, many protein-based molecular motors harness the chemical free energy released by the hydrolysis of ATP in order to perform mechanical work. In terms of energetic efficiency, this type of motor can be superior to currently available man-made motors. One important difference between molecular motors and macroscopic motors is that molecular motors operate in the thermal bath, an environment in which the fluctuations due to thermal noise are significant.

The nanocar is a molecule designed in 2005 at Rice University by a group headed by Professor James Tour. Despite the name, the original nanocar does not contain a molecular motor, hence, it is not really a car. Rather, it was designed to answer the question of how fullerenes move about on metal surfaces; specifically, whether they roll or slide.

Pentacene is a polycyclic aromatic hydrocarbon consisting of five linearly-fused benzene rings. This highly conjugated compound is an organic semiconductor. The compound generates excitons upon absorption of ultra-violet (UV) or visible light; this makes it very sensitive to oxidation. For this reason, this compound, which is a purple powder, slowly degrades upon exposure to air and light.

Synthetic molecular motors are molecular machines capable of continuous directional rotation under an energy input. Although the term "molecular motor" has traditionally referred to a naturally occurring protein that induces motion, some groups also use the term when referring to non-biological, non-peptide synthetic motors. Many chemists are pursuing the synthesis of such molecular motors.
In chemistry and molecular physics, fluxionalmolecules are molecules that undergo dynamics such that some or all of their atoms interchange between symmetry-equivalent positions. Because virtually all molecules are fluxional in some respects, e.g. bond rotations in most organic compounds, the term fluxional depends on the context and the method used to assess the dynamics. Often, a molecule is considered fluxional if its spectroscopic signature exhibits line-broadening due to chemical exchange. In some cases, where the rates are slow, fluxionality is not detected spectroscopically, but by isotopic labeling and other methods.

A Molecular propeller is a molecule that can propel fluids when rotated, due to its special shape that is designed in analogy to macroscopic propellers: it has several molecular-scale blades attached at a certain pitch angle around the circumference of a shaft, aligned along the rotational axis.
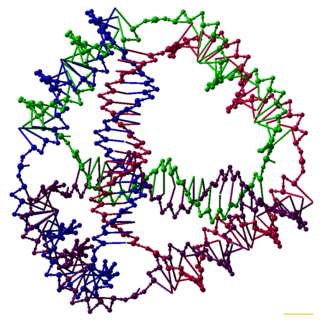
DNA nanotechnology is the design and manufacture of artificial nucleic acid structures for technological uses. In this field, nucleic acids are used as non-biological engineering materials for nanotechnology rather than as the carriers of genetic information in living cells. Researchers in the field have created static structures such as two- and three-dimensional crystal lattices, nanotubes, polyhedra, and arbitrary shapes, and functional devices such as molecular machines and DNA computers. The field is beginning to be used as a tool to solve basic science problems in structural biology and biophysics, including applications in X-ray crystallography and nuclear magnetic resonance spectroscopy of proteins to determine structures. Potential applications in molecular scale electronics and nanomedicine are also being investigated.
The single-molecule electric motor is an electrically operated synthetic molecular motor made from a single butyl methyl sulphide molecule. The molecule is adsorbed onto a copper (111) single-crystal piece by chemisorption. The motor, the world's smallest electric motor, is just a nanometer across. It was developed by the Sykes group and scientists at the Tufts University School of Arts and Sciences and published online September 4, 2011.
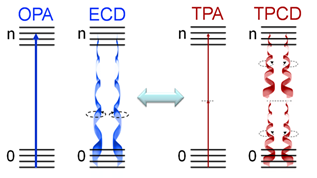
Two-photon circular dichroism (TPCD), the nonlinear counterpart of electronic circular dichroism (ECD), is defined as the differences between the two-photon absorption (TPA) cross-sections obtained using left circular polarized light and right circular polarized light.
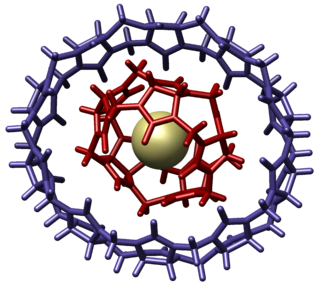
Molecular gyroscopes are chemical compounds or supramolecular complexes containing a rotor that moves freely relative to a stator, and therefore act as gyroscopes. Though any single bond or triple bond permits a chemical group to freely rotate, the compounds described as gyroscopes may protect the rotor from interactions, such as in a crystal structure with low packing density or by physically surrounding the rotor avoiding steric contact. A qualitative distinction can be made based on whether the activation energy needed to overcome rotational barriers is higher than the available thermal energy. If the activation energy required is higher than the available thermal energy, the rotor undergoes "site exchange", jumping in discrete steps between local energy minima on the potential energy surface. If there is thermal energy sufficiently higher than that needed to overcome the barrier to rotation, the molecular rotor can behave more like a macroscopic freely rotating inertial mass.

Bernard Lucas "Ben" Feringa is a Dutch synthetic organic chemist, specializing in molecular nanotechnology and homogeneous catalysis.
Azzedine Bousseksou is a Franco-Algerian physical chemist known for his contributions to molecular materials and spintronics.















