Related Research Articles

An analgesic drug, also called simply an analgesic, pain reliever, or painkiller, is any member of the group of drugs used to achieve relief from pain. It is typically used to induce cooperation with a medical procedure. Analgesics are conceptually distinct from anesthetics, which temporarily reduce, and in some instances eliminate, sensation, although analgesia and anesthesia are neurophysiologically overlapping and thus various drugs have both analgesic and anesthetic effects.

Tetrodotoxin (TTX) is a potent neurotoxin. Its name derives from Tetraodontiformes, an order that includes pufferfish, porcupinefish, ocean sunfish, and triggerfish; several of these species carry the toxin. Although tetrodotoxin was discovered in these fish and found in several other animals, it is actually produced by certain infecting or symbiotic bacteria like Pseudoalteromonas, Pseudomonas, and Vibrio as well as other species found in animals.

Amiloride, sold under the trade name Midamor among others, is a medication typically used with other medications to treat high blood pressure or swelling due to heart failure or cirrhosis of the liver. Amiloride is classified as a potassium-sparing diuretic. Amiloride is often used together with another diuretic, such as a thiazide or loop diuretic. It is taken by mouth. Onset of action is about two hours and it lasts for about a day.
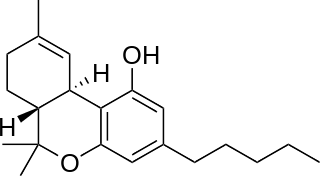
Nabiximols is a specific Cannabis extract that was approved in 2010 as a botanical drug in the United Kingdom. Nabiximols is sold as a mouth spray intended to alleviate neuropathic pain, spasticity, overactive bladder, and other symptoms of multiple sclerosis; it was developed by the UK company GW Pharmaceuticals. In 2019 it was proposed that following application of the spray, nabiximols is washed away from the oral mucosa by the saliva flow and ingested into the stomach, with subsequent absorption from the gastro-intestinal tract. Nabiximols is a combination drug standardized in composition, formulation, and dose. Its principal active cannabinoid components are the cannabinoids: tetrahydrocannabinol (THC) and cannabidiol (CBD). Each spray delivers a dose of 2.7 mg THC and 2.5 mg CBD.

4-Aminopyridine (4-AP, fampridine, dalfampridine) is an organic compound with the chemical formula C5H4N–NH2. The molecule is one of the three isomeric amines of pyridine. It is used as a research tool in characterizing subtypes of the potassium channel. It has also been used as a drug, to manage some of the symptoms of multiple sclerosis, and is indicated for symptomatic improvement of walking in adults with several variations of the disease. It was undergoing Phase III clinical trials as of 2008, and the U.S. Food and Drug Administration (FDA) approved the compound on January 22, 2010. Fampridine is also marketed as Ampyra (pronounced "am-PEER-ah," according to the maker's website) in the United States by Acorda Therapeutics and as Fampyra in the European Union, Canada, and Australia. In Canada, the medication has been approved for use by Health Canada since February 10, 2012.

Pegaptanib sodium injection is an anti-angiogenic medicine for the treatment of neovascular (wet) age-related macular degeneration (AMD). It was discovered by NeXstar Pharmaceuticals and licensed in 2000 to EyeTech Pharmaceuticals, now OSI Pharmaceuticals, for late stage development and marketing in the United States. Gilead Sciences continues to receive royalties from the drugs licensing. Outside the US pegaptanib is marketed by Pfizer. Approval was granted by the U.S. Food and Drug Administration (FDA) in December 2004.

Zomepirac is an orally effective nonsteroidal anti-inflammatory drug (NSAID) that has antipyretic actions. It was developed by McNeil Pharmaceutical, approved by the FDA in 1980, and sold as the sodium salt zomepirac sodium, under the brand name Zomax. Due to its clinical effectiveness, it was preferred by doctors in many situations and obtained a large share of the analgesics market; however, it was subsequently withdrawn in March 1983 due to its tendency to cause serious anaphylaxis in a small, but unpredictable, subset of the patient population.

Ranolazine, sold under the brand name Ranexa among others, is a medication used to treat heart related chest pain. Typically it is used together with other medications when those are insufficient. Benefits appear smaller in women than men. It is taken by mouth.

Sodium channel, voltage-gated, type XI, alpha subunit also known as SCN11A or Nav1.9 is a voltage-gated sodium ion channel protein which is encoded by the SCN11A gene on chromosome 3 in humans. Like Nav1.7 and Nav1.8, Nav1.9 plays a role in pain perception. This channel is largely expressed in small-diameter nociceptors of the dorsal root ganglion and trigeminal ganglion neurons, but is also found in intrinsic myenteric neurons.
Leronlimab is a humanized monoclonal antibody targeted against the CCR5 receptor found on T lymphocytes of the human immune system. It is being investigated as a potential therapy in the treatment of COVID-19, triple negative breast cancer, and HIV infection. The United States Food and Drug Administration has designated PRO 140 for fast-track approval. In February 2008, the drug entered Phase 2 clinical trials and a phase 3 trial was begun in 2015. In February 2018, Cytodyn Inc reported that the primary endpoint had been achieved in the PRO 140 pivotal combination therapy trial in HIV infection. In 2020 CytoDyn submitted a fast-track biologics license application for treatment of CCR5-tropic HIV-1 Infection.
Sodium channel blockers are drugs which impair the conduction of sodium ions (Na+) through sodium channels.
Chemotherapy-induced peripheral neuropathy (CIPN) is a nerve-damaging side effect of antineoplastic agents in the common cancer treatment, chemotherapy. CIPN afflicts between 30% and 40% of patients undergoing chemotherapy. Antineoplastic agents in chemotherapy are designed to eliminate rapidly dividing cancer cells, but they can also damage healthy structures, including the peripheral nervous system. CIPN involves various symptoms such as tingling, pain, and numbness in the hands and feet. These symptoms can impair activities of daily living, such as typing or dressing, reduce balance, and increase risk of falls and hospitalizations. They can also give cause to reduce or discontinue chemotherapy. Researchers have conducted clinical trials and studies to uncover the various symptoms, causes, pathogenesis, diagnoses, risk factors, and treatments of CIPN.

Neosaxitoxin (NSTX) is included, as other saxitoxin-analogs, in a broad group of natural neurotoxic alkaloids, commonly known as the paralytic shellfish toxins (PSTs). The parent compound of PSTs, saxitoxin (STX), is a tricyclic perhydropurine alkaloid, which can be substituted at various positions, leading to more than 30 naturally occurring STX analogues. All of them are related imidazoline guanidinium derivatives.

Crofelemer is an antidiarrheal indicated for the symptomatic relief of non-infectious diarrhea in adult patients with HIV/AIDS on antiretroviral therapy. Other possible uses include diarrhea in children, acute infectious diarrhea, and diarrhea in patients with irritable bowel syndrome. It is a purified oligomeric proanthocyanidin from "dragon's blood", the sap of the South American tree Croton lechleri.
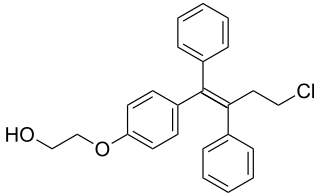
Ospemifene is an oral medication indicated for the treatment of dyspareunia – pain during sexual intercourse – encountered by some women, more often in those who are post-menopausal. Ospemifene is a selective estrogen receptor modulator (SERM) acting similarly to an estrogen on the vaginal epithelium, building vaginal wall thickness which in turn reduces the pain associated with dyspareunia. Dyspareunia is most commonly caused by "vulvar and vaginal atrophy."

Funapide (INN) is a novel analgesic under development by Xenon Pharmaceuticals for the treatment of a variety of chronic pain conditions, including osteoarthritis, neuropathic pain, postherpetic neuralgia, and erythromelalgia, as well as dental pain. It acts as a small-molecule Nav1.7 and Nav1.8 voltage-gated sodium channel blocker. Funapide is being evaluated in humans in both oral and topical formulations, and as of July 2014, has reached phase IIb clinical trials.
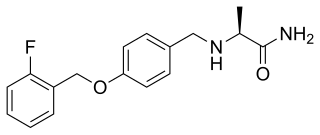
Ralfinamide (INN) is a multimodal drug which is under investigation by Newron Pharmaceuticals for the treatment of neuropathic pain and other pain conditions such as post-operative dental pain.
Evenamide (INN) is a selective voltage-gated sodium channel blocker, including subtypes Nav1.3, Nav1.7, and Nav1.8, which is described as an antipsychotic and is under development by Newron Pharmaceuticals as an add-on therapy for the treatment of schizophrenia. The drug has shown efficacy in animal models of psychosis, mania, depression, and aggression. It has completed phase I clinical trials, and phase II clinical trials will be commenced in the third quarter of 2015.
Cemiplimab, sold under the brand name Libtayo, is a monoclonal antibody medication for the treatment of squamous cell skin cancer. Cemiplimab belongs to a class of drugs that binds to the programmed death receptor-1 (PD-1), blocking the PD-1/PD-L1 pathway.
Dostarlimab, sold under the brand name Jemperli, is a monoclonal antibody used as a medication for the treatment of endometrial cancer. Dostarlimab is a programmed death receptor-1 (PD-1)–blocking monoclonal antibody.
References
- ↑ Hagen NA, Fisher KM, Lapointe B, du Souich P, Chary S, Moulin D, Sellers E, Ngoc AH (August 2007). "An open-label, multi-dose efficacy and safety study of intramuscular tetrodotoxin in patients with severe cancer-related pain". J Pain Symptom Manage. 34 (2): 171–82. doi: 10.1016/j.jpainsymman.2006.11.008 . PMID 17662911.
- ↑ "Tectin". WEX Pharmaceuticals Inc. Retrieved 2008-05-02.