Related Research Articles
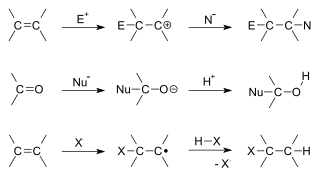
In organic chemistry, an addition reaction is an organic reaction in which two or more molecules combine to form a larger molecule called the adduct.

Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to PPh3 or Ph3P. It is versatile compound that is widely used as a reagent in organic synthesis and as a ligand for transition metal complexes, including ones that serve as catalysts in organometallic chemistry. PPh3 exists as relatively air stable, colorless crystals at room temperature. It dissolves in non-polar organic solvents such as benzene and diethyl ether.

Carbon tetrabromide, CBr4, also known as tetrabromomethane, is a bromide of carbon. Both names are acceptable under IUPAC nomenclature.

Iron(III) bromide is the chemical compound with the formula FeBr3. Also known as ferric bromide, this red-brown odorless compound is used as a Lewis acid catalyst in the halogenation of aromatic compounds. It dissolves in water to give acidic solutions.

Ditellurium bromide is the inorganic compound with the formula Te2Br. It is one of the few stable lower bromides of tellurium. Unlike sulfur and selenium, tellurium forms families of polymeric subhalides where the halide/chalcogen ratio is less than 2.

Iron(II) bromide refers to inorganic compounds with the chemical formula FeBr2(H2O)x. The anhydrous compound (x = 0) is a yellow or brownish-colored paramagnetic solid. The tetrahydrate is also known, all being pale colored solids. They are common precursor to other iron compounds.

Cadmium bromide is the inorganic compound with the formula CdBr2. It is a white hygroscopic solid. It also can be obtained as a mono- and tetrahydrate. It has few applications.

Copper(I) bromide is the chemical compound with the formula CuBr. This white diamagnetic solid adopts a polymeric structure akin to that for zinc sulfide. The compound is widely used in the synthesis of organic compounds and as a lasing medium in copper bromide lasers.
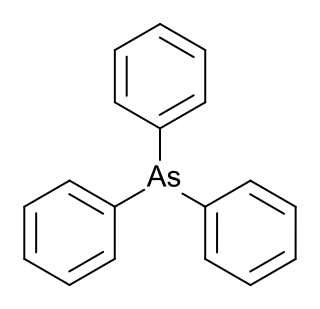
Triphenylarsine is the chemical compound with the formula As(C6H5)3. This organoarsenic compound, often abbreviated AsPh3, is a colorless crystalline solid that is used as a ligand and a reagent in coordination chemistry and organic synthesis. The molecule is pyramidal with As-C distances of 1.942–1.956 Å and C-As-C angles of 99.6–100.5°.
Magnesium bromide are inorganic compounds with the chemical formula MgBr2(H2O)x, where x can range from 0 to 9. They are all white deliquescent solids. Some magnesium bromides have been found naturally as rare minerals such as: bischofite and carnallite.

Organonickel chemistry is a branch of organometallic chemistry that deals with organic compounds featuring nickel-carbon bonds. They are used as a catalyst, as a building block in organic chemistry and in chemical vapor deposition. Organonickel compounds are also short-lived intermediates in organic reactions. The first organonickel compound was nickel tetracarbonyl Ni(CO)4, reported in 1890 and quickly applied in the Mond process for nickel purification. Organonickel complexes are prominent in numerous industrial processes including carbonylations, hydrocyanation, and the Shell higher olefin process.
Organotellurium chemistry describes the synthesis and properties of organotellurium compounds, chemical compounds containing a carbon-tellurium chemical bond. Organotellurium chemistry is a lightly studied area, in part because of it having few applications.
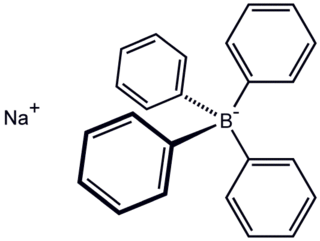
Sodium tetraphenylborate is the organic compound with the formula NaB(C6H5)4. It is a salt, wherein the anion consists of four phenyl rings bonded to boron. This white crystalline solid is used to prepare other tetraphenylborate salts, which are often highly soluble in organic solvents. The compound is used in inorganic and organometallic chemistry as a precipitating agent for potassium, ammonium, rubidium, and caesium ions, and some organic nitrogen compounds.
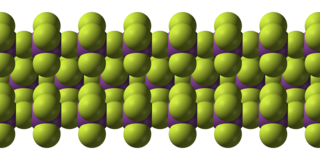
Bismuth pentafluoride is an inorganic compound with the formula BiF5. It is a white solid that is highly reactive. The compound is of interest to researchers but not of particular value.
Organoantimony chemistry is the chemistry of compounds containing a carbon to antimony (Sb) chemical bond. Relevant oxidation states are SbV and SbIII. The toxicity of antimony limits practical application in organic chemistry.

In chemistry, the pentazenium cation is a positively-charged polyatomic ion with the chemical formula N+5 and structure N−N−N−N−N. Together with solid nitrogen polymers and the azide anion, it is one of only three poly-nitrogen species obtained in bulk quantities.

Bismuth tribromide is an inorganic compound of bismuth and bromine with the chemical formula BiBr3.

Palladium(II) bromide is an inorganic compound of palladium and bromine with the chemical formula PdBr2. It is a commercially available, although less common than palladium(II) chloride, the usual entry point to palladium complexes. It is a diamagnetic solid.
Sodium hypobromite is an inorganic compound with the chemical formula NaOBr. It is a sodium salt of hypobromous acid. It consists of sodium cations Na+ and hypobromite anions −OBr. It is usually obtained as the pentahydrate, so the compound that is usually called sodium hypobromite actually has the formula NaBrO·5H2O. It is a yellow-orange solid that is soluble in water. It adopts a monoclinic crystal structure with a Br–O bond length of 1.820 Å. It is the bromine analogue of sodium hypochlorite, the active ingredient in common bleach. In practice the salt is usually encountered as an aqueous solution.
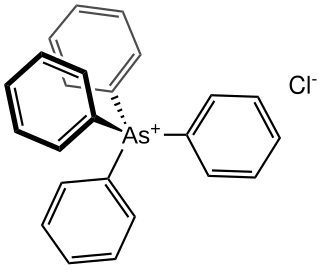
Tetraphenylarsonium chloride is the organoarsenic compound with the formula (C6H5)4AsCl. This white solid is the chloride salt of the tetraphenylarsonium cation, which is tetrahedral. Typical of related quat salts, it is soluble in polar organic solvents. It often is used as a hydrate.
References
- ↑ Jens Beckmann, Dainis Dakternieks, Andrew Duthie, François Ribot, Markus Schürmann, and Naomi A. Lewcenko "New Insights into the "Structures of Diorganotellurium Oxides. The First Polymeric Diorganotelluroxane [(p-MeOC6H4)2TeO]n" Organometallics, 2003, volume 22, 3257–3261. doi : 10.1021/om021024c