
In immunology, an antigen (Ag) is a molecule, moiety, foreign particulate matter, or an allergen, such as pollen, that can bind to a specific antibody or T-cell receptor. The presence of antigens in the body may trigger an immune response.

An antibody (Ab) or immunoglobulin (Ig) is a large, Y-shaped protein belonging to the immunoglobulin superfamily which is used by the immune system to identify and neutralize antigens such as bacteria and viruses, including those that cause disease. Antibodies can recognize virtually any size antigen with diverse chemical compositions from molecules. Each antibody recognizes one or more specific antigens. Antigen literally means "antibody generator", as it is the presence of an antigen that drives the formation of an antigen-specific antibody. Each tip of the "Y" of an antibody contains a paratope that specifically binds to one particular epitope on an antigen, allowing the two molecules to bind together with precision. Using this mechanism, antibodies can effectively "tag" a microbe or an infected cell for attack by other parts of the immune system, or can neutralize it directly.

An oncogene is a gene that has the potential to cause cancer. In tumor cells, these genes are often mutated, or expressed at high levels.

In genetics, chromosome translocation is a phenomenon that results in unusual rearrangement of chromosomes. This includes balanced and unbalanced translocation, with two main types: reciprocal, and Robertsonian translocation. Reciprocal translocation is a chromosome abnormality caused by exchange of parts between non-homologous chromosomes. Two detached fragments of two different chromosomes are switched. Robertsonian translocation occurs when two non-homologous chromosomes get attached, meaning that given two healthy pairs of chromosomes, one of each pair "sticks" and blends together homogeneously.
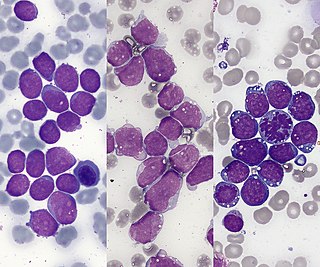
Acute lymphoblastic leukemia (ALL) is a cancer of the lymphoid line of blood cells characterized by the development of large numbers of immature lymphocytes. Symptoms may include feeling tired, pale skin color, fever, easy bleeding or bruising, enlarged lymph nodes, or bone pain. As an acute leukemia, ALL progresses rapidly and is typically fatal within weeks or months if left untreated.

Sir Gregory Paul Winter is a Nobel Prize-winning English molecular biologist best known for his work on the therapeutic use of monoclonal antibodies. His research career has been based almost entirely at the MRC Laboratory of Molecular Biology and the MRC Centre for Protein Engineering, in Cambridge, England.
V(D)J recombination is the mechanism of somatic recombination that occurs only in developing lymphocytes during the early stages of T and B cell maturation. It results in the highly diverse repertoire of antibodies/immunoglobulins and T cell receptors (TCRs) found in B cells and T cells, respectively. The process is a defining feature of the adaptive immune system.

The Medical Research Council (MRC) Laboratory of Molecular Biology (LMB) is a research institute in Cambridge, England, involved in the revolution in molecular biology which occurred in the 1950–60s. Since then it has remained a major medical research laboratory at the forefront of scientific discovery, dedicated to improving the understanding of key biological processes at atomic, molecular and cellular levels using multidisciplinary methods, with a focus on using this knowledge to address key issues in human health.
Alan James Munro is a British immunologist and entrepreneur who served as the Master of Christ's College, Cambridge (1995–2002).

Alexander J. Varshavsky is a Russian-American biochemist and geneticist. He works at the California Institute of Technology (Caltech) as the Morgan Professor of Biology. Varshavsky left Russia in 1977, emigrating to United States.
Michael Samuel Neuberger FRS FMedSci was a British biochemist and immunologist.

The Cambridge Biomedical Campus is the largest centre of medical research and health science in Europe. The site is located at the southern end of Hills Road in Cambridge, England.
Suzanne Cory is an Australian molecular biologist. She has worked on the genetics of the immune system and cancer and has lobbied her country to invest in science. She is married to fellow scientist Jerry Adams, also a WEHI scientist.
A bispecific monoclonal antibody is an artificial protein that can simultaneously bind to two different types of antigen or two different epitopes on the same antigen. Naturally occurring antibodies typically only target one antigen. BsAbs can be manufactured in several structural formats. BsAbs can be designed to recruit and activate immune cells, to interfere with receptor signaling and inactivate signaling ligands, and to force association of protein complexes. BsAbs have been explored for cancer immunotherapy, drug delivery, and Alzheimer's disease.
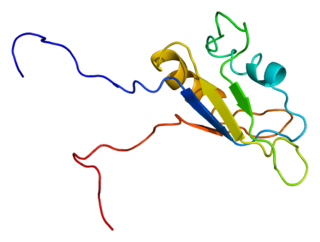
RNA-binding protein EWS is a protein that in humans is encoded by the EWSR1 gene on human chromosome 22, specifically 22q12.2. It is one of 3 proteins in the FET protein family.
The Sir William Dunn School of Pathology is a department within the University of Oxford. Its research programme includes the cellular and molecular biology of pathogens, the immune response, cancer and cardiovascular disease. It teaches undergraduate and graduate courses in the medical sciences.
Gene expression profiling has revealed that diffuse large B-cell lymphoma (DLBCL) is composed of at least 3 different sub-groups, each having distinct oncogenic mechanisms that respond to therapies in different ways. Germinal Center B-Cell like (GCB) DLBCLs appear to arise from normal germinal center B cells, while Activated B-cell like (ABC) DLBCLs are thought to arise from postgerminal center B cells that are arrested during plasmacytic differentiation. The differences in gene expression between GCB DLBCL and ABC DLBCL are as vast as the differences between distinct types of leukemia, but these conditions have historically been grouped together and treated as the same disease.
Ashok Venkitaraman is a British cancer researcher of Indian origin. He is the Director of the Cancer Science Institute of Singapore, a Distinguished Professor of Medicine at the National University of Singapore, and Program Director at A*STAR, Singapore. From 1998 to 2020, he was the inaugural holder of the Ursula Zoellner Professorship of Cancer Research at the University of Cambridge, a Professorial Fellow at Pembroke College, Cambridge, and from 2006 to 2019, was the Director of the Medical Research Council Cancer Unit.

Ketan Jayakrishna Patel is a British–Kenyan scientist who is Director of the MRC Weatherall Institute of Molecular Medicine and the MRC Molecular Haematology Unit at the University of Oxford. Until 2020 he was a tenured principal investigator at the Medical Research Council (MRC) Laboratory of Molecular Biology (LMB).

The MRC Weatherall Institute of Molecular Medicine at the University of Oxford is a research institute located at the John Radcliffe Hospital in Oxford. Founded in 1989 by Sir David Weatherall, the institute focuses on furthering our understanding of clinical medicine at a molecular level. It was one of the first institutes of its kind in the world to be dedicated to research in this area.











