
Microvilli are microscopic cellular membrane protrusions that increase the surface area for diffusion and minimize any increase in volume, and are involved in a wide variety of functions, including absorption, secretion, cellular adhesion, and mechanotransduction.

In cell biology, the cleavage furrow is the indentation of the cell's surface that begins the progression of cleavage, by which animal and some algal cells undergo cytokinesis, the final splitting of the membrane, in the process of cell division. The same proteins responsible for muscle contraction, actin and myosin, begin the process of forming the cleavage furrow, creating an actomyosin ring. Other cytoskeletal proteins and actin binding proteins are involved in the procedure.

A myofibril is a basic rod-like unit of a muscle cell. Muscles are composed of tubular cells called myocytes, known as muscle fibers in striated muscle, and these cells in turn contain many chains of myofibrils. They are created during embryonic development in a process known as myogenesis.
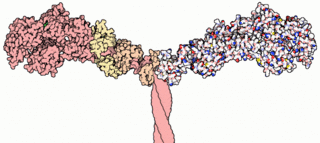
Myosins are a superfamily of motor proteins best known for their roles in muscle contraction and in a wide range of other motility processes in eukaryotes. They are ATP-dependent and responsible for actin-based motility. The term was originally used to describe a group of similar ATPases found in the cells of both striated muscle tissue and smooth muscle tissue. Following the discovery by Pollard and Korn (1973) of enzymes with myosin-like function in Acanthamoeba castellanii, a global range of divergent myosin genes have been discovered throughout the realm of eukaryotes.
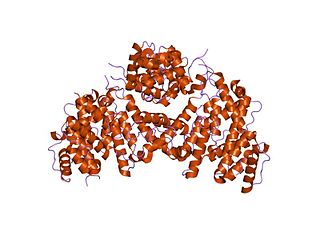
Fimbrin also known as is plastin 1 is a protein that in humans is encoded by the PLS1 gene. Fimbrin is an actin cross-linking protein important in the formation of filopodia.
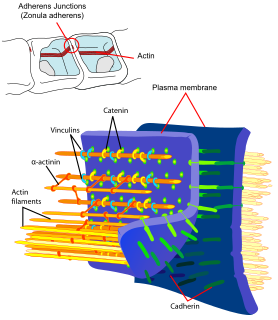
Adherens junctions are protein complexes that occur at cell–cell junctions in epithelial and endothelial tissues, usually more basal than tight junctions. An adherens junction is defined as a cell junction whose cytoplasmic face is linked to the actin cytoskeleton. They can appear as bands encircling the cell or as spots of attachment to the extracellular matrix . Adherens junctions uniquely disassemble in uterine epithelial cells to allow the blastocyst to penetrate between epithelial cells.
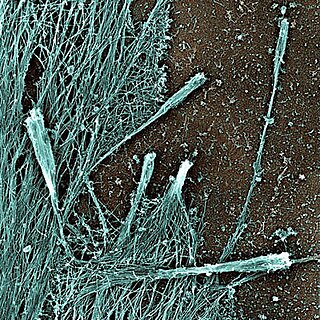
Filopodia are slender cytoplasmic projections that extend beyond the leading edge of lamellipodia in migrating cells. They contain actin filaments cross-linked into bundles by actin-binding proteins, e.g. fascin and fimbrin. Filopodia form focal adhesions with the substratum, linking it to the cell surface. Many types of migrating cells display filopodia, which are thought to be involved in both sensation of chemotropic cues, and resulting changes in directed locomotion.
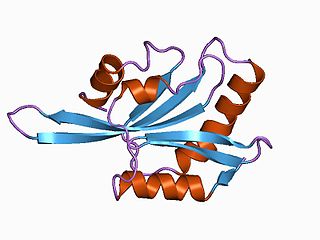
ADF/cofilin is a family of actin-binding proteins which disassembles actin filaments. Three highly conserved and highly (70%-82%) identical genes belonging to this family have been described in humans and mice:

In cell biology, a bleb is a bulge or protrusion of the plasma membrane of a cell, human bioparticulate or abscess with an internal environment similar to that of a simple cell, characterized by a spherical, bulky morphology. It is characterized by the decoupling of the cytoskeleton from the plasma membrane, degrading the internal structure of the cell, allowing the flexibility required to allow the cell to separate into individual bulges or pockets of the intercellular matrix. Most commonly, blebs are seen in apoptosis but are also seen in other non-apoptotic functions. Blebbing, or zeiosis, is the formation of blebs.

Alpha-catenin was proposed to function as a linking protein between cadherins and actin-containing filaments of the cytoskeleton. It has been reported that the actin binding proteins vinculin and alpha-actinin can bind to alpha-catenin. However, a protein complex including a cadherin, actin, beta-catenin and alpha-catenin has not been isolated. It has been suggested that alpha-catenin does not bind with high affinity to both actin filaments and the E-cadherin-beta-catenin complex at the same time. It has been observed that when alpha-catenin is not in a molecular complex with beta-catenin, it dimerizes and functions to regulate actin filament assembly, possibly by competing with Arp2/3 protein. Alpha catenin exhibits significant protein dynamics.

Sodium-hydrogen antiporter 3 regulator 1 is a regulator of Sodium-hydrogen antiporter 3. It is encoded by the gene SLC9A3R1. It is also known as ERM Binding Protein 50 (EBP50) or Na+/H+ Exchanger Regulatory Factor (NHERF1). It is believed to interact via long-range allostery, involving significant protein dynamics.
In enzymology, a myosin-heavy-chain kinase is an enzyme that catalyzes the chemical reaction

Myosin-Ic is a protein that in humans is encoded by the MYO1C gene.

Myosin-Ia is a protein that in humans is encoded by the MYO1A gene.

Stress fibers are contractile actin bundles found in non-muscle cells. They are composed of actin (microfilaments) and non-muscle myosin II (NMMII), and also contain various crosslinking proteins, such as α-actinin, to form a highly regulated actomyosin structure within non-muscle cells. Stress fibers have been shown to play an important role in cellular contractility, providing force for a number of functions such as cell adhesion, migration and morphogenesis.

The ERM protein family consists of three closely related proteins, ezrin, radixin and moesin. The three paralogs, ezrin, radixin and moesin, are present in vertebrates, whereas other species have only one ERM gene. Therefore, in vertebrates these paralogs likely arose by gene duplication.

Rho-associated protein kinase (ROCK) is a kinase belonging to the AGC family of serine-threonine kinases. It is involved mainly in regulating the shape and movement of cells by acting on the cytoskeleton.
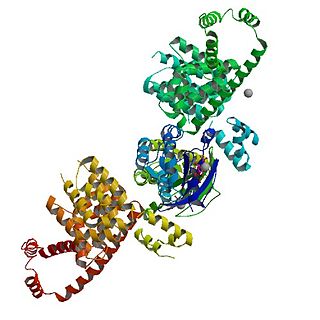
mDia1 is a member of the protein family called the formins and is a Rho effector. It is the mouse version of the diaphanous homolog 1 of Drosophila. mDia1 localizes to cells' mitotic spindle and midbody, plays a role in stress fiber and filopodia formation, phagocytosis, activation of serum response factor, formation of adherens junctions, and it can act as a transcription factor. mDia1 accelerates actin nucleation and elongation by interacting with barbed ends of actin filaments. The gene encoding mDia1 is located on Chromosome 18 of Mus musculus and named Diap1.
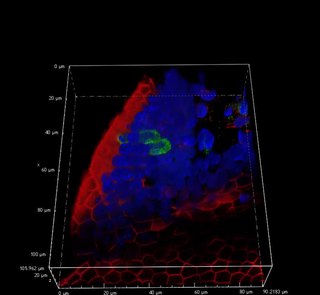
Tuft cells, sometimes referred to as brush cells, are chemosensory cells in the epithelial lining of the intestines and respiratory tract. The names "tuft" and "brush" refer to the microvilli projecting from the cells.



















