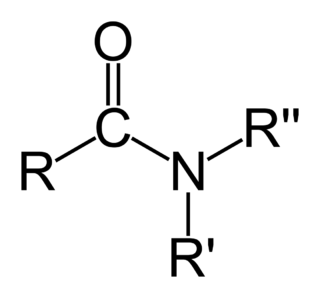
In organic chemistry, an amide ( or or, also known as an organic amide or a carboxamide, is a compound with the general formula RC NR′R″, where R, R', and R″ represent organic groups or hydrogen atoms. The amide group is called a peptide bond when it is part of the main chain of a protein, and an isopeptide bond when it occurs in a side chain, such as in the amino acids asparagine and glutamine. It can be viewed as a derivative of a carboxylic acid RC OH with the hydroxyl group –OH replaced by an amine group –NR′R″; or, equivalently, an acyl group RC – joined to an amine group.

An ester is a chemical compound derived from an acid in which at least one –OH hydroxyl group is replaced by an –O– alkyl (alkoxy) group, as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides are fatty acid esters of glycerol; they are important in biology, being one of the main classes of lipids and comprising the bulk of animal fats and vegetable oils.

Acetoacetic acid is the organic compound with the formula CH3COCH2COOH. It is the simplest beta-keto acid, and like other members of this class, it is unstable. The methyl and ethyl esters, which are quite stable, are produced on a large scale industrially as precursors to dyes. Acetoacetic acid is a weak acid.

In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containing a carbonyl group is often referred to as a carbonyl compound.

Fluorescein is an organic compound and dye. It is available as a dark orange/red powder slightly soluble in water and alcohol. It is widely used as a fluorescent tracer for many applications.
In biochemistry, biotinylation is the process of covalently attaching biotin to a protein, nucleic acid or other molecule. Biotinylation is rapid, specific and is unlikely to disturb the natural function of the molecule due to the small size of biotin. Biotin binds to streptavidin and avidin with an extremely high affinity, fast on-rate, and high specificity, and these interactions are exploited in many areas of biotechnology to isolate biotinylated molecules of interest. Biotin-binding to streptavidin and avidin is resistant to extremes of heat, pH and proteolysis, making capture of biotinylated molecules possible in a wide variety of environments. Also, multiple biotin molecules can be conjugated to a protein of interest, which allows binding of multiple streptavidin, avidin or neutravidin protein molecules and increases the sensitivity of detection of the protein of interest. There is a large number of biotinylation reagents available that exploit the wide range of possible labelling methods. Due to the strong affinity between biotin and streptavidin, the purification of biotinylated proteins has been a widely used approach to identify protein-protein interactions and post-translational events such as ubiquitylation in molecular biology.

Rhodamine is a family of related dyes, a subset of the triarylmethane dyes. They are derivatives of xanthene. Important members of the rhodamine family are Rhodamine 6G, Rhodamine 123, and Rhodamine B. They are mainly used to dye paper and inks, but they lack the lightfastness for fabric dying.

Fluorescein isothiocyanate (FITC) is a derivative of fluorescein used in wide-ranging applications including flow cytometry. First described in 1942, FITC is the original fluorescein molecule functionalized with an isothiocyanate reactive group (−N=C=S), replacing a hydrogen atom on the bottom ring of the structure. It is typically available as a mixture of isomers, fluorescein 5-isothiocyanate (5-FITC) and fluorescein 6-isothiocyanate (6-FITC). FITC is reactive towards nucleophiles including amine and sulfhydryl groups on proteins. It was synthesized by Robert Seiwald and Joseph Burckhalter in 1958.
Cyanines, also referred to as tetramethylindo(di)-carbocyanines are defined as "synthetic dyes with the general formula R2N[CH=CH]nCH=N+R2↔R2N+=CH[CH=CH]nNR2 in which the nitrogen and part of the conjugated chain usually form part of a heterocyclic system, such as imidazole, pyridine, pyrrole, quinoline and thiazole." Cyanines are used in industry biotechnology.

Trimethylsilyl chloride, also known as chlorotrimethylsilane is an organosilicon compound (silyl halide), with the formula (CH3)3SiCl, often abbreviated Me3SiCl or TMSCl. It is a colourless volatile liquid that is stable in the absence of water. It is widely used in organic chemistry.

6-Carboxyfluorescein (6-FAM) is a fluorescent dye with an absorption wavelength of 495 nm and an emission wavelength of 517 nm. A carboxyfluorescein molecule is a fluorescein molecule with a carboxyl group added. They are commonly used as a tracer agents. It is used in the sequencing of nucleic acids and in the labeling of nucleotides.
Carbamic acid, which might also be called aminoformic acid or aminocarboxylic acid, is the chemical compound with the formula H2NCOOH. It can be obtained by the reaction of ammonia NH3 and carbon dioxide CO2 at very low temperatures, which also yields an equal amount of ammonium carbamate. The compound is stable only up to about 250 K (−23 °C); at higher temperatures it decomposes into those two gases. The solid apparently consists of dimers, with the two molecules connected by hydrogen bonds between the two carboxyl groups –COOH.

Carboxyfluorescein succinimidyl ester (CFSE) is a fluorescent cell staining dye. CFSE is cell permeable and covalently couples, via its succinimidyl group, to intracellular molecules, notably, to intracellular lysine residues and other amine sources. Due to this covalent coupling reaction, fluorescent CFSE can be retained within cells for extremely long periods. Also, due to this stable linkage, once incorporated within cells, the dye is not transferred to adjacent cells.

Carboxyfluorescein diacetate succinimidyl ester (CFDA-SE) is a cell permeable dye generally used in animal cell proliferation research. High concentrations of the dye are toxic to animal cells, however concentrations in the region of 10 micromolar are typically sufficient to give strong staining with minimal cell death.
Bissulfosuccinimidyl suberate (BS3) is a crosslinker used in biological research. It is a water-soluble version of disuccinimidyl suberate.
The Ei mechanism, also known as a thermal syn elimination or a pericyclic syn elimination, in organic chemistry is a special type of elimination reaction in which two vicinal substituents on an alkane framework leave simultaneously via a cyclic transition state to form an alkene in a syn elimination. This type of elimination is unique because it is thermally activated and does not require additional reagents unlike regular eliminations which require an acid or base, or would in many cases involve charged intermediates. This reaction mechanism is often found in pyrolysis.

Cypenamine, or cypenamine hydrochloride (USAN), also known as 2-phenylcyclopentylamine, is a psychostimulant drug which was developed by a group at the William S. Merrell Chemical Company in the 1940s. It is currently known only in scientific research and has never been developed for market use. Cypenamine is currently legal throughout the entire world, and though its chemical structure has a vague similarity to certain controlled stimulants like fencamfamine, it is likely that it is too distant for it to be considered an illicit analogue under the United States Federal Analogue Act of the Controlled Substances Act.

Sunepitron is a combined 5-HT1A receptor agonist and α2-adrenergic receptor antagonist. It was previously under development by Pfizer for the treatment of depression and anxiety. It made it to phase III clinical trials before being discontinued.

Pentafluorophenyl (PFP) esters are chemical compounds with the generic formula RC(O)OC6F5. They are active esters derived from pentafluorophenol (HOC6F5).

Succinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate (SMCC) is a heterobifunctional amine-to-sulfhydryl crosslinker, which contains two reactive groups at opposite ends: N-hydroxysuccinimide-ester and maleimide, reactive with amines and thiols respectively. SMCC is often used in bioconjugation to link proteins with other functional entities. For example, a targeted anticancer agent – trastuzumab emtansine – is prepared using SMCC reagent.














