Related Research Articles

In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or in the terminal position. Terminal alkenes are also known as α-olefins.

Cis–trans isomerism, also known as geometric isomerism, describes certain arrangements of atoms within molecules. The prefixes "cis" and "trans" are from Latin: "this side of" and "the other side of", respectively. In the context of chemistry, cis indicates that the functional groups (substituents) are on the same side of some plane, while trans conveys that they are on opposing (transverse) sides. Cis–trans isomers are stereoisomers, that is, pairs of molecules which have the same formula but whose functional groups are in different orientations in three-dimensional space. Cis and trans isomers occur both in organic molecules and in inorganic coordination complexes. Cis and trans descriptors are not used for cases of conformational isomerism where the two geometric forms easily interconvert, such as most open-chain single-bonded structures; instead, the terms "syn" and "anti" are used.

In stereochemistry, stereoisomerism, or spatial isomerism, is a form of isomerism in which molecules have the same molecular formula and sequence of bonded atoms (constitution), but differ in the three-dimensional orientations of their atoms in space. This contrasts with structural isomers, which share the same molecular formula, but the bond connections or their order differs. By definition, molecules that are stereoisomers of each other represent the same structural isomer.

Laser cutting is a technology that uses a laser to vaporize materials, resulting in a cut edge. While typically used for industrial manufacturing applications, it is now used by schools, small businesses, architecture, and hobbyists. Laser cutting works by directing the output of a high-power laser most commonly through optics. The laser optics and CNC are used to direct the laser beam to the material. A commercial laser for cutting materials uses a motion control system to follow a CNC or G-code of the pattern to be cut onto the material. The focused laser beam is directed at the material, which then either melts, burns, vaporizes away, or is blown away by a jet of gas, leaving an edge with a high-quality surface finish.
But-2-ene is an acyclic alkene with four carbon atoms. It is the simplest alkene exhibiting cis/trans-isomerism (also known as (E/Z)-isomerism); that is, it exists as two geometric isomers cis-but-2-ene ((Z)-but-2-ene) and trans-but-2-ene ((E)-but-2-ene).

(E)-Stilbene, commonly known as trans-stilbene, is an organic compound represented by the condensed structural formula C6H5CH=CHC6H5. Classified as a diarylethene, it features a central ethylene moiety with one phenyl group substituent on each end of the carbon–carbon double bond. It has an (E) stereochemistry, meaning that the phenyl groups are located on opposite sides of the double bond, the opposite of its geometric isomer, cis-stilbene. Trans-stilbene occurs as a white crystalline solid at room temperature and is highly soluble in organic solvents. It can be converted to cis-stilbene photochemically, and further reacted to produce phenanthrene.
Cycloocta-1,5-diene is a cyclic hydrocarbon with the chemical formula C8H12, specifically [−(CH2)2−CH=CH−]2.
Cis or cis- may refer to:
Perfluorodecalin is a fluorocarbon, a derivative of decalin in which all of the hydrogen atoms are replaced by fluorine atoms. It is chemically and biologically inert and stable up to 400 °C. Several applications make use of its ability to dissolve gases.
Diimide, also called diazene or diimine, is a compound having the formula HN=NH. It exists as two geometric isomers, E (trans) and Z (cis). The term diazene is more common for organic derivatives of diimide. Thus, azobenzene is an example of an organic diazene.
Stilbene may refer to one of the two stereoisomers of 1,2-diphenylethene:

2,3,3,3-Tetrafluoropropene, HFO-1234yf, is a hydrofluoroolefin (HFO) with the formula CH2=CFCF3. It is also designated R-1234yf as the first of a new class of refrigerants: it is marketed under the name Opteon YF by Chemours and as Solstice YF by Honeywell. R-1234yf is also a component of zeotropic refrigerant blend R-454B, an alternative to the R-410A refrigerant currently used in air conditioning and heat pump applications.

Hydrofluoroolefins (HFOs) are unsaturated organic compounds composed of hydrogen, fluorine and carbon. These organofluorine compounds are of interest as refrigerants. Unlike traditional hydrofluorocarbons (HFCs) and chlorofluorocarbons (CFCs), which are saturated, HFOs are olefins, otherwise known as alkenes.

trans-1,3,3,3-Tetrafluoropropene (HFO-1234ze(E), R-1234ze(E)) is a hydrofluoroolefin. It was developed as a "fourth generation" refrigerant to replace fluids such as R-134a, as a blowing agent for foam and aerosol applications, and in air horns and gas dusters. The use of R-134a is being phased out because of its high global warming potential (GWP). HFO-1234ze(E) itself has zero ozone-depletion potential (ODP=0), a very low global warming potential (GWP < 1 ), even lower than CO2, and it is classified by ANSI/ASHRAE as class A2L refrigerant (lower flammability and lower toxicity).
1,3,3,3-Tetrafluoropropene is a hydrofluoroolefin which has two isomers:

Cyclononene is a cycloalkene with a nine-membered ring, with two possible geometric isomers, denoted cis-cyclononene and trans-cyclononene, or (Z)-cyclononene and (E)-cyclononene.
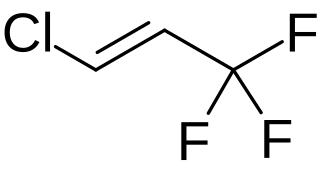
1-Chloro-3,3,3-trifluoropropene (HFO-1233zd) is the unsaturated chlorofluorocarbon with the formula HClC=C(H)CF3. The compound exists as E- (cis-) and Z- (trans-) isomers. The trans- isomer of this colorless gas is of interest as a more environmentally friendly (lower GWP; global warming potential) refrigerant in air conditioners. It is prepared by fluorination and dehydrohalogenation reactions starting with 1,1,1,3,3-pentachloropropane.
Diphenylethylene or Diphenylethene may refer to:

Cidoxepin (former developmental code name P-4599), also known as cis-doxepin or (Z)-doxepin, is a tricyclic antidepressant which was developed in the 1960s but was never marketed. It is the cis or (Z) stereoisomer of doxepin, a mixture of (E) and (Z) isomers that is used commercially in a ratio of approximately 85:15 with cidoxepin as a relatively minor constituent. However, the drug has similar activity to that of doxepin, acting as a serotonin–norepinephrine reuptake inhibitor, H1 receptor antagonist, and anticholinergic, and notably is thought to have more antidepressant activity than trans-doxepin. The central anticholinergic activity of cidoxepin has been reported to be 3-fold greater than that of the trans isomer in mice.
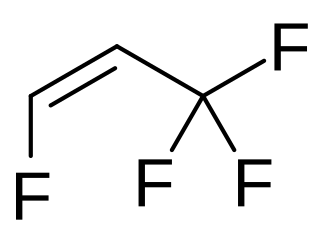
cis-1,3,3,3-Tetrafluoropropene is a hydrofluoroolefin which has been considered for use as a refrigerant in high-temperature heat pumps.
References
- ↑ "Laser cutting teflon". Universal Laser Systems, Inc.