Related Research Articles

Growth hormone (GH) or somatotropin, also known as human growth hormones in its human form, is a peptide hormone that stimulates growth, cell reproduction, and cell regeneration in humans and other animals. It is thus important in human development. GH also stimulates production of IGF-1 and increases the concentration of glucose and free fatty acids. It is a type of mitogen which is specific only to the receptors on certain types of cells. GH is a 191-amino acid, single-chain polypeptide that is synthesized, stored and secreted by somatotropic cells within the lateral wings of the anterior pituitary gland.
Congenital adrenal hyperplasia (CAH) is a group of autosomal recessive disorders characterized by impaired cortisol synthesis. It results from the deficiency of one of the five enzymes required for the synthesis of cortisol in the adrenal cortex. Most of these disorders involve excessive or deficient production of such hormones as glucocorticoids, mineralocorticoids, or sex steroids, and can alter development of primary or secondary sex characteristics in some affected infants, children or adults. It is one of the most common autosomal recessive disorders in humans.

Growth hormone deficiency (GHD) is a medical condition resulting from not enough growth hormone (GH). Generally the most noticeable symptom is that an individual attains a short height. Newborns may also present low blood sugar or a small penis size. In adults there may be decreased muscle mass, high cholesterol levels, or poor bone density.
Growth hormone therapy refers to the use of growth hormone (GH) as a prescription medication—it is one form of hormone therapy. Growth hormone is a peptide hormone secreted by the pituitary gland that stimulates growth and cell reproduction. In the past, growth hormone was extracted from human pituitary glands. Growth hormone is now produced by recombinant DNA technology and is prescribed for a variety of reasons. GH therapy has been a focus of social and ethical controversies for 50 years.
Delayed puberty is when a person lacks or has incomplete development of specific sexual characteristics past the usual age of onset of puberty. The person may have no physical or hormonal signs that puberty has begun. In the United States, girls are considered to have delayed puberty if they lack breast development by age 13 or have not started menstruating by age 16. Boys are considered to have delayed puberty if they lack enlargement of the testicles by age 14. Delayed puberty affects about 2% of adolescents.
Controversies regarding the use of human growth hormone (HGH) as treatment method have centered on the claims, products, and businesses related to the use of growth hormone as an anti-aging therapy. Most of these controversies fall into two categories:
- Claims of exaggerated, misleading, or unfounded assertions that growth hormone treatment safely and effectively slows or reverses the effects of aging.
- The sale of products that fraudulently or misleadingly purport to be growth hormone or to increase the user's own secretion of natural human growth hormone to a beneficial degree.
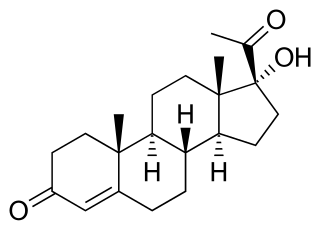
Congenital adrenal hyperplasia due to 21-hydroxylase deficiency in all its forms, accounts for over 95% of diagnosed cases of congenital adrenal hyperplasia (CAH), and CAH in most contexts refers to 21-hydroxylase deficiency and different mutations related to enzyme impairment have been mapped on protein structure.
Saizen is a commercial preparation of synthetic somatropin. Manufactured by Merck Serono, Saizen is produced by recombinant DNA technology from a mammalian cell line that was modified by the addition of the human GH gene, resulting in an identical 191-amino acid sequence and structure.

Laron syndrome (LS), also known as growth hormone insensitivity is an autosomal recessive disorder characterized by a lack of insulin-like growth factor 1 (IGF-1)(somatomedin) production in response to growth hormone (GH)(hGH)(somatotropin). It is usually caused by inherited growth hormone receptor (GHR) mutations.
CJC-1295, also known as DAC:GRF, is a synthetic analogue of growth hormone-releasing hormone (GHRH) and a growth hormone secretagogue (GHS) which was developed by ConjuChem Biotechnologies. It is a modified form of GHRH (1-29) with improved pharmacokinetics, especially in regard to half-life.

Ibutamoren (INN) is a potent, long-acting, orally-active, selective, and non-peptide agonist of the ghrelin receptor and a growth hormone secretagogue, mimicking the growth hormone (GH)-stimulating action of the endogenous hormone ghrelin. It has been shown to increase the secretion of several hormones including GH and insulin-like growth factor 1 (IGF-1) and produces sustained increases in the plasma levels of these hormones without affecting cortisol levels.

Tabimorelin (INN) is a drug which acts as a potent, orally-active agonist of the ghrelin/growth hormone secretagogue receptor (GHSR) and growth hormone secretagogue, mimicking the effects of the endogenous peptide agonist ghrelin as a stimulator of growth hormone (GH) release. It was one of the first GH secretagogues developed and is largely a modified polypeptide, but it is nevertheless orally-active in vivo. Tabimorelin produced sustained increases in levels of GH and insulin-like growth factor 1 (IGF-1), along with smaller transient increases in levels of other hormones such as adrenocorticotropic hormone (ACTH), cortisol, and prolactin. However actual clinical effects in adults with growth hormone deficiency were limited, with only the most severely GH-deficient patients showing significant benefit, and tabimorelin was also found to act as a CYP3A4 inhibitor which could cause it to have undesirable interactions with other drugs.
The anti-aging movement is a social movement devoted to eliminating or reversing aging, or reducing the effects of it. A substantial portion of the attention of the movement is on the possibilities for life extension, but there is also interest in techniques such as cosmetic surgery which ameliorate the effects of aging rather than delay or defeat it.

Kowarski syndrome describes cases of growth failure, despite the presence of normal or slightly high blood growth hormone by radioimmunoassay (RIA-GH) and low serum IGF1, and who exhibit a significant increase in growth rate following recombinant GH therapy.
The Cambridge Pulmonary Hypertension Outcome Review (CAMPHOR) is a disease specific patient-reported outcome measure which assesses quality of life of patients with pulmonary hypertension (PH). It was the first pulmonary hypertension specific questionnaire for assessing patient reported symptoms, quality of life and functioning.
The Patient Reported Outcome Indices for Multiple Sclerosis (PRIMUS) is a disease specific patient-reported outcome questionnaire which measures the quality of life (QoL) of patients suffering from multiple sclerosis.
The Recurrent Genital Herpes Quality of Life (RGHQoL) measure is a patient-reported outcome measure which determines the impact that recurrent genital herpes has on a patient’s quality of life. It is a 20 item questionnaire with items such as “Herpes makes it difficult for me to plan ahead” and “I worry that sex will trigger an outbreak.”. Lower scores on the RGHQoL indicate a higher negative impact on quality of life.
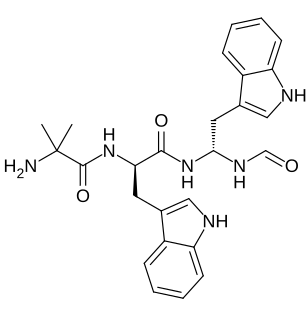
Macimorelin (INN), or Macrilen is a drug being developed by Æterna Zentaris for use in the diagnosis of adult growth hormone deficiency. Macimorelin acetate, the salt formulation, is a synthetic growth hormone secretagogue receptor agonist. Macimorelin acetate is described chemically as D-Tryptophanamide, 2-methylalanyl-N-[(1R)-1-(formylamino)-2-(1H-indol-3-yl)ethyl]-acetate.
Somapacitan, sold under the brand name Sogroya, is a growth hormone medication. Somapacitan is a human growth hormone analog. Somapacitan-beco is produced in Escherichia coli by recombinant DNA technology.
References
- 1 2 Wirén, L.; Whalley, D; McKenna, S; Wilhelmsen, L (2000). "Application of a disease-specific, quality-of-life measure (QoL-AGHDA) in growth hormone-deficient adults and a random population sample in Sweden: Validation of the measure by Rasch analysis". Clinical Endocrinology. 52 (2): 143–52. doi:10.1046/j.1365-2265.2000.00899.x. PMID 10671940.
- 1 2 Hull, K.; Harvey, S (2003). "Growth hormone therapy and Quality of Life: Possibilities, pitfalls and mechanisms". Journal of Endocrinology. 179 (3): 311–33. doi: 10.1677/joe.0.1790311 . PMID 14656202.
- ↑ "QoL-AGHDA: Quality of Life: Assessment of GH Deficiency in Adults" (PDF). Pharmacia. 2000. Retrieved 27 September 2013.
- ↑ McKenna, Stephen P.; Doward, Lynda C.; Alonso, Jordi; Kohlmann, Thomas; Niero, Mauro; Prieto, Luis; Wíren, Lena (1999). "The QoL-AGHDA: An instrument for the assessment of quality of life in adults with growth hormone deficiency". Quality of Life Research. 8 (4): 373–83. doi:10.1023/A:1008987922774. PMID 10472170.
- ↑ "Measures Database". Galen-Research.com. Galen Research. Retrieved 27 September 2013.[ non-primary source needed ]
- 1 2 McKenna, Stephen P; Wilburn, Jeanette; Twiss, James; Crawford, Sigrid R; Hána, Václav; Karbownik-Lewinska, Malgorzata; Popovic, Vera; Pura, Mikulas; Koltowska-Häggström, Maria (2011). "Adaptation of the QoL-AGHDA scale for adults with growth hormone deficiency in four Slavic languages". Health and Quality of Life Outcomes. 9: 60. doi:10.1186/1477-7525-9-60. PMC 3199740 . PMID 21810234.
- ↑ Moock, Joern; Friedrich, Nele; Völzke, Henry; Spielhagen, Christin; Nauck, Matthias; Koltowska-Häggström, Maria; Buchfelder, Michael; Wallaschofski, Henri; Kohlmann, Thomas (2011). "Prediction of improvement in quality of life (QoL-AGHDA) in adults with growth hormone deficiency by normative reference limits: Data of the German KIMS cohort". Growth Hormone & IGF Research. 21 (5): 272–8. doi:10.1016/j.ghir.2011.07.005. PMID 21865066.
- ↑ Gilet, Hélène; Chachuat, Anne; Viala-Danten, Muriel; Auzière, Sébastien; Koltowska-Häggström, Maria (2010). "Application of the Disease-Specific Quality of Life Assessment of Growth Hormone Deficiency in Adults (QoL-AGHDA) Questionnaire in a General Population: Results from a French Panel Study". Value in Health. 13 (4): 495–500. doi: 10.1111/j.1524-4733.2009.00689.x . PMID 20102556.
- ↑ Badia, X.; Lucas, A.; Sanmartí, A.; Roset, M.; Ulied, A. (1998). "One-year follow-up of quality of life in adults with untreated growth hormone deficiency". Clinical Endocrinology. 49 (6): 765–71. doi:10.1046/j.1365-2265.1998.00634.x. PMID 10209564.
- ↑ Mukherjee, A.; Tolhurst-Cleaver, S; Ryder, WD; Smethurst, L; Shalet, SM (2004). "The Characteristics of Quality of Life Impairment in Adult Growth Hormone (GH)-Deficient Survivors of Cancer and Their Response to GH Replacement Therapy". Journal of Clinical Endocrinology & Metabolism. 90 (3): 1542–9. doi: 10.1210/jc.2004-0832 . PMID 15613427.
- ↑ Gutiérrez, Lia P.; Kołtowska-Häggström, Maria; Jönsson, Peter J.; Mattsson, Anders F.; Svensson, Dag; Westberg, Björn; Luger, Anton (2008). "Registries as a tool in evidence-based medicine: Example of KIMS (Pfizer International Metabolic Database)". Pharmacoepidemiology and Drug Safety. 17 (1): 90–102. doi:10.1002/pds.1510. PMID 17957812.
- ↑ "Evidence and interpretation". Human growth hormone (somatropin) in adults with growth hormone deficiency. National Institute for Health and Care Excellence. August 2003. p. 17. ISBN 978-1-84257-356-3 . Retrieved 27 September 2013.