Related Research Articles

Adhesive, also known as glue, cement, mucilage, or paste, is any non-metallic substance applied to one or both surfaces of two separate items that binds them together and resists their separation.

The chemical industry comprises the companies and other organizations that develop and produce industrial, specialty and other chemicals. Central to the modern world economy, it converts raw materials into commodity chemicals for industrial and consumer products. It includes industries for petrochemicals such as polymers for plastics and synthetic fibers; inorganic chemicals such as acids and alkalis; agricultural chemicals such as fertilizers, pesticides and herbicides; and other categories such as industrial gases, speciality chemicals and pharmaceuticals.
In chemistry, a disulfide is a compound containing a R−S−S−R′ functional group or the S2−
2 anion. The linkage is also called an SS-bond or sometimes a disulfide bridge and usually derived from two thiol groups.
A period 3 element is one of the chemical elements in the third row of the periodic table of the chemical elements. The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behavior of the elements as their atomic number increases: a new row is begun when chemical behavior begins to repeat, meaning that elements with similar behavior fall into the same vertical columns. The third period contains eight elements: sodium, magnesium, aluminium, silicon, phosphorus, sulfur, chlorine and argon. The first two, sodium and magnesium, are members of the s-block of the periodic table, while the others are members of the p-block. All of the period 3 elements occur in nature and have at least one stable isotope.

Epoxy is the family of basic components or cured end products of epoxy resins. Epoxy resins, also known as polyepoxides, are a class of reactive prepolymers and polymers which contain epoxide groups. The epoxide functional group is also collectively called epoxy. The IUPAC name for an epoxide group is an oxirane.
Sulfur dyes are the most commonly used dyes manufactured for cotton in terms of volume. They are inexpensive, generally have good wash-fastness, and are easy to apply. Sulfur dyes are predominantly black, brown, and dark blue. Red sulfur dyes are unknown, although a pink or lighter scarlet color is available.
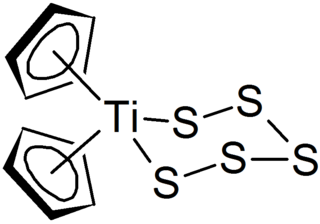
Polysulfides are a class of chemical compounds derived from anionic chains of sulfur atoms. There are two main classes of polysulfides: inorganic and organic. The inorganic polysulfides have the general formula S2−
n. These anions are the conjugate bases of polysulfanes H2Sn. Organic polysulfides generally have the formulae R1SnR2, where R is an alkyl or aryl group.
Accelerants, or accelerators, are substances that increase the rate of a natural or artificial chemical process. They play a major role in chemistry, as most chemical reactions can be hastened with an accelerant. Understanding accelerants is crucial in forensic science, engineering, and other fields where controlled chemical reactions are essential. Accelerants function by either altering a chemical bond, speeding up a chemical process, or changing the reaction conditions. Unlike catalysts, accelerants may be consumed during the process.
Thiokol was an American corporation concerned initially with rubber and related chemicals, and later with rocket and missile propulsion systems. Its name is a portmanteau of the Greek words for sulfur and glue, an allusion to the company's initial product, Thiokol polymer.
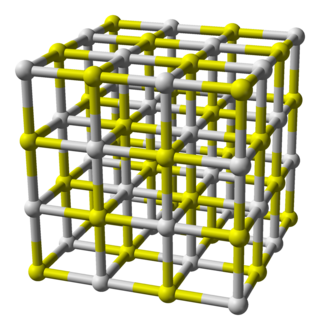
Calcium sulfide is the chemical compound with the formula CaS. This white material crystallizes in cubes like rock salt. CaS has been studied as a component in a process that would recycle gypsum, a product of flue-gas desulfurization. Like many salts containing sulfide ions, CaS typically has an odour of H2S, which results from small amount of this gas formed by hydrolysis of the salt.
Sulfur compounds are chemical compounds formed the element sulfur (S). Common oxidation states of sulfur range from −2 to +6. Sulfur forms stable compounds with all elements except the noble gases.
Silicone rubber is an elastomer composed of silicone—itself a polymer—containing silicon together with carbon, hydrogen, and oxygen. Silicone rubbers are widely used in industry, and there are multiple formulations. Silicone rubbers are often one- or two-part polymers, and may contain fillers to improve properties or reduce cost. Silicone rubber is generally non-reactive, stable, and resistant to extreme environments and temperatures from −55 to 300 °C while still maintaining its useful properties. Due to these properties and its ease of manufacturing and shaping, silicone rubber can be found in a wide variety of products, including voltage line insulators; automotive applications; cooking, baking, and food storage products; apparel such as undergarments, sportswear, and footwear; electronics; medical devices and implants; and in home repair and hardware, in products such as silicone sealants.
Sealant is a substance used to block the passage of fluids through openings in materials, a type of mechanical seal. In building construction sealant is sometimes synonymous with caulk and also serve the purposes of blocking dust, sound and heat transmission. Sealants may be weak or strong, flexible or rigid, permanent or temporary. Sealants are not adhesives but some have adhesive qualities and are called adhesive-sealants or structural sealants.
A pyrotechnic composition is a substance or mixture of substances designed to produce an effect by heat, light, sound, gas/smoke or a combination of these, as a result of non-detonative self-sustaining exothermic chemical reactions. Pyrotechnic substances do not rely on oxygen from external sources to sustain the reaction.
Curing is a chemical process employed in polymer chemistry and process engineering that produces the toughening or hardening of a polymer material by cross-linking of polymer chains. Even if it is strongly associated with the production of thermosetting polymers, the term "curing" can be used for all the processes where a solid product is obtained from a liquid solution, such as with PVC plastisols.
The polysulfide–bromine battery, is a type of rechargeable electric battery, which stores electric energy in liquids, such as water-based solutions of two salts: sodium bromide and sodium polysulfide. It is an example and type of redox (reduction–oxidation) flow battery.
RTV silicone is a type of silicone rubber that cures at room temperature. It is available as a one-component product, or mixed from two components. Manufacturers provide it in a range of hardnesses from very soft to medium—usually from 15 to 40 Shore A. RTV silicones can be cured with a catalyst consisting of either platinum or a tin compound such as dibutyltin dilaurate. Applications include low-temperature over-molding, making molds for reproducing, and lens applications for some optically clear grades. It is also used widely in the automotive industry as an adhesive and sealant, for example to create gaskets in place.

Sodium polysulfide is a general term for salts with the formula Na2Sx, where x = 2 to 5. The species Sx2−, called polysulfide anions, include disulfide (S22−), trisulfide (S32−), tetrasulfide (S42−), and pentasulfide (S52−). In principle, but not in practice, the chain lengths could be longer. The salts are dark red solids that dissolve in water to give highly alkaline and corrosive solutions. In air, these salts oxidize, and they evolve hydrogen sulfide by hydrolysis.
Sodium tetrasulfide is an inorganic compound with the formula Na2S4. It is a yellow-orange solid that dissolves via hydrolysis in water. It is a precursor to some specialty polymers and intermediates in prototypes of the sodium-sulfur battery.
2-Ethylhexyl glycidyl ether is a liquid organic molecule with formula C11H22O2 an industrial chemical used to reduce the viscosity of epoxy resins. These are then used in adhesives, sealants, and paints or coatings. It has the CAS Registry Number of 2461-15-6. It has the IUPAC name of 2-(2-ethylhexoxymethyl)oxirane. It also finds use in other polymer based applications.
References
- ↑ Mark S. M. Alger (1997). Polymer Science Dictionary. Springer. p. 569. ISBN 978-0-412-60870-4.
- 1 2 3 Vietti, David; Scherrer, Micheal (2000). "Polymers Containing Sulfur, Polysulfides". Kirk-Othmer Encyclopedia of Chemical Technology. New York: John Wiley. doi:10.1002/0471238961.1615122522090520.a01. ISBN 9780471238966.