Related Research Articles

Cysteine (symbol Cys or C; ) is a semiessential proteinogenic amino acid with the formula HOOC-CH-(NH2)-CH2-SH. The thiol side chain in cysteine often participates in enzymatic reactions as a nucleophile. The thiol is susceptible to oxidation to give the disulfide derivative cystine, which serves an important structural role in many proteins. When used as a food additive, it has the E number E920. It is encoded by the codons UGU and UGC.
In biochemistry, a disulfide refers to a functional group with the structure R−S−S−R′. The linkage is also called an SS-bond or sometimes a disulfide bridge and is usually derived by the coupling of two thiol groups. In biology, disulfide bridges formed between thiol groups in two cysteine residues are an important component of the secondary and tertiary structure of proteins. Persulfide usually refers to R−S−S−H compounds.

Glutathione (GSH) is an antioxidant in plants, animals, fungi, and some bacteria and archaea. Glutathione is capable of preventing damage to important cellular components caused by reactive oxygen species such as free radicals, peroxides, lipid peroxides, and heavy metals. It is a tripeptide with a gamma peptide linkage between the carboxyl group of the glutamate side chain and cysteine. The carboxyl group of the cysteine residue is attached by normal peptide linkage to glycine.

Metallothionein (MT) is a family of cysteine-rich, low molecular weight proteins. They are localized to the membrane of the Golgi apparatus. MTs have the capacity to bind both physiological and xenobiotic heavy metals through the thiol group of its cysteine residues, which represent nearly 30% of its constituent amino acid residues.
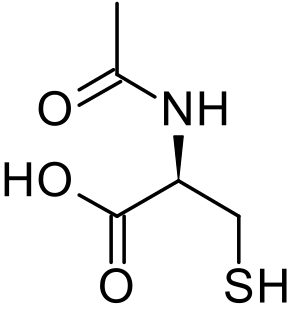
Acetylcysteine, also known as N-acetylcysteine (NAC), is a medication that is used to treat paracetamol (acetaminophen) overdose, and to loosen thick mucus in individuals with chronic bronchopulmonary disorders like pneumonia and bronchitis. It has been used to treat lactobezoar in infants. It can be taken intravenously, by mouth, or inhaled as a mist. Some people use it as a dietary supplement.
Protein disulfide isomerase, or PDI, is an enzyme in the endoplasmic reticulum (ER) in eukaryotes and the periplasm of bacteria that catalyzes the formation and breakage of disulfide bonds between cysteine residues within proteins as they fold. This allows proteins to quickly find the correct arrangement of disulfide bonds in their fully folded state, and therefore the enzyme acts to catalyze protein folding.

Thioredoxin is a class of small redox proteins known to be present in all organisms. It plays a role in many important biological processes, including redox signaling. In humans, thioredoxins are encoded by TXN and TXN2 genes. Loss-of-function mutation of either of the two human thioredoxin genes is lethal at the four-cell stage of the developing embryo. Although not entirely understood, thioredoxin is linked to medicine through their response to reactive oxygen species (ROS). In plants, thioredoxins regulate a spectrum of critical functions, ranging from photosynthesis to growth, flowering and the development and germination of seeds. Thioredoxins play a role in cell-to-cell communication.

Cysteine dioxygenase (CDO) is a non-heme iron enzyme that catalyzes the conversion of L-cysteine to cysteine sulfinic acid. CDO plays an important role in cysteine catabolism, regulating intracellular levels of cysteine and responding changes in cysteine availability. As such, CDO is highly regulated and undergoes large changes in concentration and efficiency. It oxidizes cysteine to the corresponding sulfinic acid by activation of dioxygen, although the exact mechanism of the reaction is still unclear. In addition to being found in mammals, CDO also exists in some yeast and bacteria, although the exact function is still unknown. CDO has been implicated in various neurodegenerative diseases and cancers, which is likely related to cysteine toxicity.
Iron–sulfur proteins are proteins characterized by the presence of iron–sulfur clusters containing sulfide-linked di-, tri-, and tetrairon centers in variable oxidation states. Iron–sulfur clusters are found in a variety of metalloproteins, such as the ferredoxins, as well as NADH dehydrogenase, hydrogenases, coenzyme Q – cytochrome c reductase, succinate – coenzyme Q reductase and nitrogenase. Iron–sulfur clusters are best known for their role in the oxidation-reduction reactions of electron transport in mitochondria and chloroplasts. Both Complex I and Complex II of oxidative phosphorylation have multiple Fe–S clusters. They have many other functions including catalysis as illustrated by aconitase, generation of radicals as illustrated by SAM-dependent enzymes, and as sulfur donors in the biosynthesis of lipoic acid and biotin. Additionally, some Fe–S proteins regulate gene expression. Fe–S proteins are vulnerable to attack by biogenic nitric oxide, forming dinitrosyl iron complexes. In most Fe–S proteins, the terminal ligands on Fe are thiolate, but exceptions exist.

A sulfenic acid is an organosulfur compound and oxoacid with the general formula RSOH. It is the first member of the family of organosulfur oxoacids, which also include sulfinic acids and sulfonic acids, RSO2H and RSO3H, respectively. The base member of the sulfenic acid series with R = H is hydrogen thioperoxide.

Cystathionine-β-synthase, also known as CBS, is an enzyme (EC 4.2.1.22) that in humans is encoded by the CBS gene. It catalyzes the first step of the transsulfuration pathway, from homocysteine to cystathionine:
Zinc deficiency is defined either as insufficient zinc to meet the needs of the body, or as a serum zinc level below the normal range. However, since a decrease in the serum concentration is only detectable after long-term or severe depletion, serum zinc is not a reliable biomarker for zinc status. Common symptoms include increased rates of diarrhea. Zinc deficiency affects the skin and gastrointestinal tract; brain and central nervous system, immune, skeletal, and reproductive systems.

Thiosulfate dehydrogenase is an enzyme that catalyzes the chemical reaction:
Glutamate Cysteine Ligase (GCL), previously known as gamma-glutamylcysteine synthetase (GCS), is the first enzyme of the cellular glutathione (GSH) biosynthetic pathway that catalyzes the chemical reaction:

Metallothionein-3 is a protein that in humans is encoded by the MT3 gene. It is a 68-amino acid peptide that is abnormally under-expressed in the brains of patients suffering from Alzheimer's disease. Metallothionein-3 is a member of the metallothionein family of proteins.

Metal regulatory transcription factor 1 is a protein that in humans is encoded by the MTF1 gene.
Glutaredoxin 2 (GLRX2) is an enzyme that in humans encoded by the GLRX2 gene. GLRX2, also known as GRX2, is a glutaredoxin family protein and a thiol-disulfide oxidoreductase that maintains cellular thiol homeostasis. This gene consists of four exons and three introns, spanned 10 kilobase pairs, and localized to chromosome 1q31.2–31.3.

γ -L-Glutamyl-L-cysteine, also known as γ-glutamylcysteine (GGC), is a dipeptide found in animals, plants, fungi, some bacteria, and archaea. It has a relatively unusual γ-bond between the constituent amino acids, L-glutamic acid and L-cysteine and is a key intermediate in the gamma (γ) -glutamyl cycle first described by Meister in the 1970s. It is the most immediate precursor to the antioxidant glutathione.

The reduction-oxidation sensitive green fluorescent protein (roGFP) is a green fluorescent protein engineered to be sensitive to changes in the local redox environment. roGFPs are used as redox-sensitive biosensors.
Cysteine-rich proteins are small proteins that contain a large number of cysteines. These cysteines either cross-link to form disulphide bonds, or bind metal ions by chelation, stabilising the protein's tertiary structure. CRPs include a highly conserved secretion peptide signal at the N-terminus and a cysteine-rich region at the C-terminus.
References
- 1 2 Wolfgang m (2003). "Cellular zinc and redox states converge in the metallothionein/thionein pair". Journal of Nutrition . 133 (5 Suppl 1): 1460S–142SS. doi:10.1093/jn/133.5.1460S. PMID 12730443.
- ↑ Palacios O, Atrian S, Capdevila, M (2011). "Zn- and Cu-thioneins: a functional classification for metallothioneins?". Journal of Biological Inorganic Chemistry . 16 (7): 991–1009. doi:10.1007/s00775-011-0827-2. PMID 21823038. S2CID 26786966.