Related Research Articles

Crohn's disease is a type of inflammatory bowel disease (IBD) that may affect any segment of the gastrointestinal tract. Symptoms often include abdominal pain, diarrhea, fever, abdominal distension, and weight loss. Complications outside of the gastrointestinal tract may include anemia, skin rashes, arthritis, inflammation of the eye, and fatigue. The skin rashes may be due to infections as well as pyoderma gangrenosum or erythema nodosum. Bowel obstruction may occur as a complication of chronic inflammation, and those with the disease are at greater risk of colon cancer and small bowel cancer.

Ulcerative colitis (UC) is one of the two types of inflammatory bowel disease (IBD), with the other type being Crohn's disease. It is a long-term condition that results in inflammation and ulcers of the colon and rectum. The primary symptoms of active disease are abdominal pain and diarrhea mixed with blood (hematochezia). Weight loss, fever, and anemia may also occur. Often, symptoms come on slowly and can range from mild to severe. Symptoms typically occur intermittently with periods of no symptoms between flares. Complications may include abnormal dilation of the colon (megacolon), inflammation of the eye, joints, or liver, and colon cancer.

The Takeda Pharmaceutical Company Limited is a Japanese multinational pharmaceutical company. It is the third largest pharmaceutical company in Asia, behind Sinopharm and Shanghai Pharmaceuticals, and one of the top 20 largest pharmaceutical companies in the world by revenue. The company has over 49,578 employees worldwide and achieved US$19.299 billion in revenue during the 2018 fiscal year. The company is focused on oncology, rare diseases, neuroscience, gastroenterology, plasma-derived therapies and vaccines. Its headquarters is located in Chuo-ku, Osaka, and it has an office in Nihonbashi, Chuo, Tokyo. In January 2012, Fortune Magazine ranked the Takeda Oncology Company as one of the 100 best companies to work for in the United States. As of 2015, Christophe Weber was appointed as the CEO and president of Takeda.

Infliximab, a chimeric monoclonal antibody, sold under the brand name Remicade among others, is a medication used to treat a number of autoimmune diseases. This includes Crohn's disease, ulcerative colitis, rheumatoid arthritis, ankylosing spondylitis, psoriasis, psoriatic arthritis, and Behçet's disease. It is given by slow injection into a vein, typically at six- to eight-week intervals.
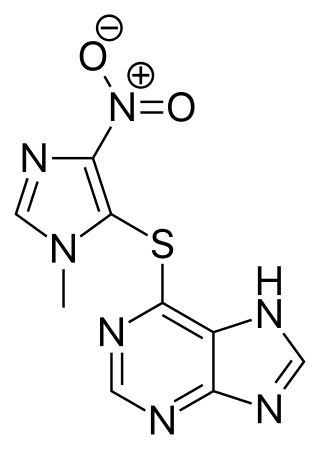
Azathioprine, sold under the brand name Imuran, among others, is an immunosuppressive medication. It is used for the treatment of rheumatoid arthritis, granulomatosis with polyangiitis, Crohn's disease, ulcerative colitis, and systemic lupus erythematosus; and in kidney transplants to prevent rejection. It is listed by the International Agency for Research on Cancer as a group 1 human carcinogen. It is taken by mouth or injected into a vein.
Hematopoietic stem-cell transplantation (HSCT) is the transplantation of multipotent hematopoietic stem cells, usually derived from bone marrow, peripheral blood, or umbilical cord blood, in order to replicate inside a patient and produce additional normal blood cells. HSCT may be autologous, syngeneic, or allogeneic.

Graft-versus-host disease (GvHD) is a syndrome, characterized by inflammation in different organs. GvHD is commonly associated with bone marrow transplants and stem cell transplants.

Inflammatory bowel disease (IBD) is a group of inflammatory conditions of the colon and small intestine, with Crohn's disease and ulcerative colitis (UC) being the principal types. Crohn's disease affects the small intestine and large intestine, as well as the mouth, esophagus, stomach and the anus, whereas UC primarily affects the colon and the rectum.

Elan Corporation plc was a major drugs firm based in Dublin, Ireland, which had major interests in the United States. It was listed on the New York Stock Exchange as ELN, the Irish Stock Exchange as ELN.I, and the London Stock Exchange as ELN.L. In 2013, the company merged with Perrigo to form Perrigo Company PLC.

Colitis is swelling or inflammation of the large intestine (colon). Colitis may be acute and self-limited or long-term. It broadly fits into the category of digestive diseases.
Management of Crohn's disease involves first treating the acute symptoms of the disease, then maintaining remission. Since Crohn's disease is an immune system condition, it cannot be cured by medication or surgery. Treatment initially involves the use of medications to eliminate infections and reduce inflammation. Surgery may be required for complications such as obstructions, fistulae, abscesses, or if the disease does not respond to drugs within a reasonable time. However, surgery cannot cure Crohn's disease. It involves removing the diseased part of the intestine and rejoining the healthy ends, but the disease tends to recur after surgery.

Biological therapy, the use of medications called biopharmaceuticals or biologics that are tailored to specifically target an immune or genetic mediator of disease, plays a major role in the treatment of inflammatory bowel disease. Even for diseases of unknown cause, molecules that are involved in the disease process have been identified, and can be targeted for biological therapy. Many of these molecules, which are mainly cytokines, are directly involved in the immune system. Biological therapy has found a niche in the management of cancer, autoimmune diseases, and diseases of unknown cause that result in symptoms due to immune related mechanisms.
Vedolizumab, sold under the brand name Entyvio, is a monoclonal antibody medication developed by Takeda Oncology for the treatment of ulcerative colitis and Crohn's disease. It binds to integrin α4β7, blocking the α4β7 integrin results in gut-selective anti-inflammatory activity.
Sir Marc Feldmann,, is an Australian-educated British immunologist. He is a professor at the University of Oxford and a senior research fellow at Somerville College, Oxford.

Maribavir, sold under the brand name Livtencity, is an antiviral medication that is used to treat post-transplant cytomegalovirus (CMV). Maribavir is a cytomegalovirus pUL97 kinase inhibitor that works by preventing the activity of human cytomegalovirus enzyme pUL97, thus blocking virus replication.

Lineage Cell Therapeutics, Inc. is a clinical-stage biotechnology company developing novel cell therapies for unmet medical needs. Lineage’s programs are based on its robust proprietary cell-based therapy platform and associated in-house development and manufacturing capabilities. With this platform Lineage develops and manufactures specialized, terminally differentiated human cells from its pluripotent and progenitor cell starting materials. These differentiated cells are developed to either replace or support cells that are dysfunctional or absent due to degenerative disease or traumatic injury or administered as a means of helping the body mount an effective immune response to cancer.
Mesoblast Limited is an Australian regenerative medicine company. It seeks to provide treatments for inflammatory ailments, cardiovascular disease, and back pain. The company is led by Silviu Itescu, who founded the company in 2004.
Crohn's disease (CD) of the vulva is a rare extra intestinal condition, with granulomatous cutaneous lesions affecting the female genitalia. Lesions connected to the affected gut via a healthy tissue are referred to as metastatic lesions.

Shimon Slavin is an Israeli professor of medicine. He pioneered immunotherapy mediated by allogeneic donor lymphocytes and innovative methods for stem cell transplantation to cure hematological malignancies and solid tumors. He also used hematopoietic stem cells to induce transplantation tolerance to bone marrow and organ allografts.
Darvadstrocel, sold under the brand name Alofisel, is a medication used to treat complex perianal fistulas in adults with non-active/mildly active luminal Crohn's disease when fistulas have shown an inadequate response to at least one conventional or biologic therapy. It contains mesenchymal stem cells from fat tissue of adult donors.
References
- ↑ "The Lancet of publishes 24-Week Results of the Phase 3 ADMIRE-CD Trial Investigating Cx601 in the Treatment of Complex Perianal Fistulas in Patients with Crohn's Disease".
{{cite journal}}: Cite journal requires|journal=(help) - ↑ "Takeda to Acquire TiGenix for €520M". 5 January 2018.