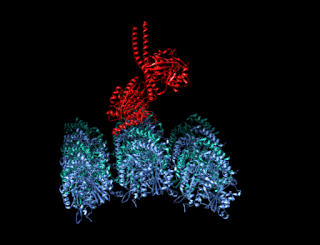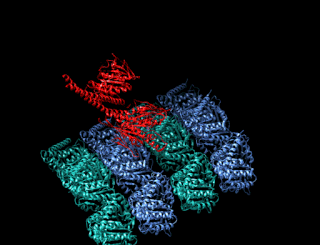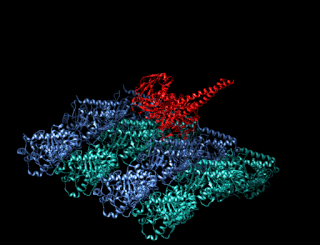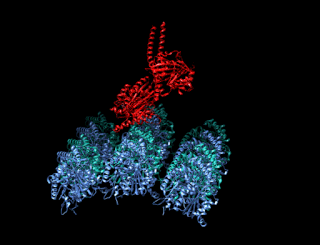
A kinesin is a protein belonging to a class of motor proteins found in eukaryotic cells.

Dynein is a family of cytoskeletal motor proteins that move along microtubules in cells. They convert the chemical energy stored in ATP to mechanical work. Dynein transports various cellular cargos, provides forces and displacements important in mitosis, and drives the beat of eukaryotic cilia and flagella. All of these functions rely on dynein's ability to move towards the minus-end of the microtubules, known as retrograde transport, thus, they are called "minus-end directed motors". In contrast, most kinesin motor proteins move toward the microtubules' plus end.

Molecular motors are natural (biological) or artificial molecular machines that are the essential agents of movement in living organisms. In general terms, a motor is a device that consumes energy in one form and converts it into motion or mechanical work; for example, many protein-based molecular motors harness the chemical free energy released by the hydrolysis of ATP in order to perform mechanical work. In terms of energetic efficiency, this type of motor can be superior to currently available man-made motors. One important difference between molecular motors and macroscopic motors is that molecular motors operate in the thermal bath, an environment in which the fluctuations due to thermal noise are significant.
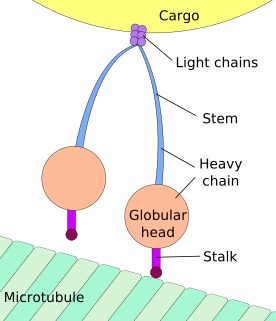
Axonal transport, also called axoplasmic transport or axoplasmic flow, is a cellular process responsible for movement of mitochondria, lipids, synaptic vesicles, proteins, and other organelles to and from a neuron's cell body, through the cytoplasm of its axon called the axoplasm. Since some axons are on the order of meters long, neurons cannot rely on diffusion to carry products of the nucleus and organelles to the end of their axons. Axonal transport is also responsible for moving molecules destined for degradation from the axon back to the cell body, where they are broken down by lysosomes.

Motor proteins are a class of molecular motors that can move along the cytoplasm of animal cells. They convert chemical energy into mechanical work by the hydrolysis of ATP. Flagellar rotation, however, is powered by a proton pump.
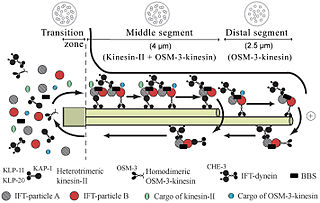
Intraflagellar transport or IFT is a bidirectional motility along axonemal microtubules that is essential for the formation (ciliogenesis) and maintenance of most eukaryotic cilia and flagella. It is thought to be required to build all cilia that assemble within a membrane projection from the cell surface. Plasmodium falciparum cilia and the sperm flagella of Drosophila are examples of cilia that assemble in the cytoplasm and do not require IFT. The process of IFT involves movement of large protein complexes called IFT particles or trains from the cell body to the ciliary tip and followed by their return to the cell body. The outward or anterograde movement is powered by kinesin-2 while the inward or retrograde movement is powered by cytoplasmic dynein 2/1b. The IFT particles are composed of about 20 proteins organized in two subcomplexes called complex A and B.

Molecular biophysics is a rapidly evolving interdisciplinary area of research that combines concepts in physics, chemistry, engineering, mathematics and biology. It seeks to understand biomolecular systems and explain biological function in terms of molecular structure, structural organization, and dynamic behaviour at various levels of complexity. This discipline covers topics such as the measurement of molecular forces, molecular associations, allosteric interactions, Brownian motion, and cable theory. Additional areas of study can be found on Outline of Biophysics. The discipline has required development of specialized equipment and procedures capable of imaging and manipulating minute living structures, as well as novel experimental approaches.

Dynactin is a 23 subunit protein complex that acts as a co-factor for the microtubule motor cytoplasmic dynein-1. It is built around a short filament of actin related protein-1 (Arp1).

Dual specificity mitogen-activated protein kinase kinase 7, also known as MAP kinase kinase 7 or MKK7, is an enzyme that in humans is encoded by the MAP2K7 gene. This protein is a member of the mitogen-activated protein kinase kinase family. The MKK7 protein exists as six different isoforms with three possible N-termini and two possible C-termini.

Kinesin-like protein KIF23 is a protein that in humans is encoded by the KIF23 gene.

Kinesin-like protein KIF2C is a protein that in humans is encoded by the KIF2C gene.

Kinesin family member 3B (KIF3B) is a protein that in humans is encoded by the KIF3B gene. KIF3B is an N-type protein that complexes with two other kinesin proteins to form two-headed anterograde motors. First, KIF3B forms a heterodimer with KIF3A (kinesin family member 3A;, that is membrane-bound and has ATPase activity. Then KIFAP3 binds to the tail domain to form a heterotrimeric motor. This motor has a plus end-directed microtubule sliding activity that exhibits a velocity of ∼0.3 μm/s a. There are 14 kinesin protein families and KIF3B is part of the Kinesin-2 family, of kinesins that can all form heterotrimeric complexes. Expression of the three motor subunits is ubiquitous. The KIG3A/3B/KAP3 motors can transport 90 to 160 nm in diameter organelles.

Kinesin light chain 3 is a protein that in humans is encoded by the KLC3 gene.
The Kinesin 8 Family are a subfamily of the molecular motor proteins known as kinesins. Most kinesins transport materials or cargo around the cell while traversing along microtubule polymer tracks with the help of ATP-hydrolysis-created energy. The Kinesin 8 family has been shown to play an important role in chromosome alignment during mitosis. Kinesin 8 family members KIF18A in humans and Kip3 in yeast have been shown to be in vivo plus-end directed microtubule depolymerizers. During prometaphase of mitosis, the microtubules attach to the kinetochores of sister chromatids. Kinesin 8 is thought to play some role in this process, as knockdown of this protein via siRNA produces a phenotype of sister chromatids that are unable to align properly.

Kinesin-like protein KIF1A, also known as axonal transporter of synaptic vesicles or microtubule-based motor KIF1A, is a protein that in humans is encoded by the KIF1A gene.
Johan Paulsson is a Swedish mathematician and systems biologist at Harvard Medical School. He is a leading researcher in systems biology and stochastic processes, specializing in stochasticity in gene networks and plasmid reproduction.

Kinesin-5 is a molecular motor protein that is essential in mitosis. Kinesin-5 proteins are members of kinesin superfamily, which are nanomotors that move along microtubule tracks in the cell. Named from studies in the early days of discovery, it is also known as kinesin family member 11, encoded by the KIF11 gene, or as BimC, Eg5 or N-2, based on the founding members of this kinesin family. The term kinesin-5 has been recommended based on a standardized nomenclature adopted by the scientific community.

Kinesin family member 15 is a protein that in humans is encoded by the KIF15 gene.
Force Spectrum Microscopy (FSM) is an application of active microrheology developed to measure aggregate random forces in the cytoplasm. Large, inert flow tracers are injected into live cells and become lodged inside the cytoskeletal mesh, wherein it is oscillated by repercussions from active motor proteins. The magnitude of these random forces can be inferred from the frequency of oscillation of tracer particles. Tracking the fluctuations of tracer particles using optical microscopy can isolate the contribution of active random forces to intracellular molecular transport from that of Brownian motion.

Intracellular transport is the movement of vesicles and substances within a cell. Intracellular transport is required for maintaining homeostasis within the cell by responding to physiological signals. Proteins synthesized in the cytosol are distributed to their respective organelles, according to their specific amino acid’s sorting sequence. Eukaryotic cells transport packets of components to particular intracellular locations by attaching them to molecular motors that haul them along microtubules and actin filaments. Since intracellular transport heavily relies on microtubules for movement, the components of the cytoskeleton play a vital role in trafficking vesicles between organelles and the plasma membrane by providing mechanical support. Through this pathway, it is possible to facilitate the movement of essential molecules such as membrane‐bounded vesicles and organelles, mRNA, and chromosomes.














