In chemistry, a structural isomer of a compound is another compound whose molecule has the same number of atoms of each element, but with logically distinct bonds between them. The term metamer was formerly used for the same concept.
1,4-Dichlorobenzene (1,4-DCB, p-DCB, or para-dichlorobenzene, sometimes abbreviated as PDCB or para) is an organic compound with the formula C6H4Cl2. This colorless solid has a strong odor. The molecule consists of a benzene ring with two chlorine atoms (replacing hydrogen atoms) on opposing sites of the ring.
Mesitylene or 1,3,5-trimethylbenzene is a derivative of benzene with three methyl substituents positioned symmetrically around the ring. The other two isomeric trimethylbenzenes are 1,2,4-trimethylbenzene (pseudocumene) and 1,2,3-trimethylbenzene (hemimellitene). All three compounds have the formula C6H3(CH3)3, which is commonly abbreviated C6H3Me3. Mesitylene is a colorless liquid with sweet aromatic odor. It is a component of coal tar, which is its traditional source. It is a precursor to diverse fine chemicals. The mesityl group (Mes) is a substituent with the formula C6H2Me3 and is found in various other compounds.

Tautomers are structural isomers of chemical compounds that readily interconvert. The chemical reaction interconverting the two is called tautomerization. This conversion commonly results from the relocation of a hydrogen atom within the compound. The phenomenon of tautomerization is called tautomerism, also called desmotropism. Tautomerism is for example relevant to the behavior of amino acids and nucleic acids, two of the fundamental building blocks of life.

Triazines are a class of nitrogen-containing heterocycles. The parent molecules' molecular formula is C3H3N3. They exist in three isomeric forms, 1,3,5-triazines being common.
A chlorophenol is any organochloride of phenol that contains one or more covalently bonded chlorine atoms. There are five basic types of chlorophenols and 19 different chlorophenols in total when positional isomerism is taken into account. Chlorophenols are produced by electrophilic halogenation of phenol with chlorine.

Disulfur dichloride is the inorganic compound of sulfur and chlorine with the formula S2Cl2. It is an amber oily liquid.
The chlorobenzenes are a family of covalent compounds consisting of one or more chlorine atoms as substituents on a benzene core. Depending on the number of chlorine substituents, there may be several constitutional isomers possible.
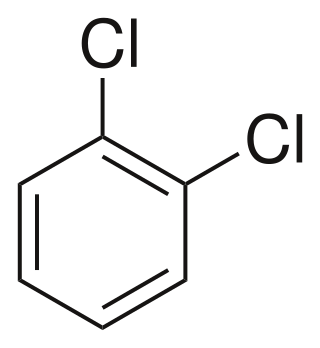
1,2-Dichlorobenzene, or orthodichlorobenzene (ODCB), is an organic compound with the formula C6H4Cl2. This colourless liquid is poorly soluble in water but miscible with most organic solvents. It is a derivative of benzene, consisting of two adjacent chlorine atoms.
Chlorinated paraffins (CPs) are complex mixtures of polychlorinated n-alkanes. The chlorination degree of CPs can vary between 30 and 70 wt%. CPs are subdivided according to their carbon chain length into short-chain CPs, medium-chain CPs and long-chain CPs. Depending on chain length and chlorine content, CPs are colorless or yellowish liquids or solids.

Trimesic acid, also known as benzene-1,3,5-tricarboxylic acid, is an organic compound with the formula C6H3(CO2H)3. It is one of three isomers of benzenetricarboxylic acid. A colorless solid, trimesic acid has some commercial value as a precursor to some plasticizers.
1,2,4-Trichlorobenzene is an organochlorine compound, one of three isomers of trichlorobenzene. It is a derivative of benzene with three chloride substituents. It is a colorless liquid used as a solvent for a variety of compounds and materials.

Hexachlorobutadiene, (often abbreviated as "HCBD") Cl2C=C(Cl)C(Cl)=CCl2, is a colorless liquid at room temperature that has an odor similar to that of turpentine. It is a chlorinated aliphatic diene with niche applications but is most commonly used as a solvent for other chlorine-containing compounds. Structurally, it has a 1,3-butadiene core, but fully substituted with chlorine atoms.
A substance of very high concern (SVHC) is a chemical substance concerning which it has been proposed that use within the European Union be subject to authorisation under the REACH Regulation. Indeed, listing of a substance as an SVHC by the European Chemicals Agency (ECHA) is the first step in the procedure for authorisation or restriction of use of a chemical. The first list of SVHCs was published on 28 October 2008 and the list has been updated many times to include new candidates. The most recent update occurred in January 2022 to include a total of 223 SVHC.
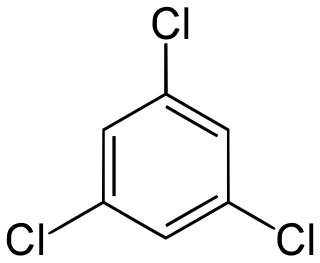
1,3,5-Trichlorobenzene is an organochlorine compound. It is one of the three isomers of trichlorobenzene. Being more symmetrical than the other isomers, it exists as colourless crystals whereas the other isomers are liquids at room temperature.

Trimellitic acid (benzene-1,2,4-tricarboxylic acid) is a chemical compound with the molecular formula C6H3(СООН)3. Like the other isomers of benzenetricarboxylic acid, trimellitic acid is a colorless solid. It is prepared by oxidation of 1,2,4-trimethylbenzene.

1,3-Dichlorobenzene (also known as meta-dichlorobenzene) is an organic compound with the formula C6H4Cl2. It is the least common of the three isomers of dichlorobenzene, and it is a colorless liquid that is insoluble in water. It is produced as a minor byproduct of the chlorination of benzene, but can also be prepared in a directed manner by the Sandmeyer reaction of 3-chloroaniline. It also arises from the isomerization of the other dichlorobenzenes at high temperature.

Hemimellitic acid (benzene-1,2,3-tricarboxylic acid) is an organic compound with the molecular formula C6H3(СО2Н)3. Like the other isomers of benzenetricarboxylic acid, hemimellitic acid is a colorless solid. It is prepared by oxidation of 1,2,3-trimethylbenzene.
1,2,3-Trichlorobenzene is an organochlorine compound with the chemical formula C6H3Cl3. This is one of three isomers of trichlorobenzene; the two others are 1,2,4-Trichlorobenzene and 1,3,5-Trichlorobenzene.
Tetrachlorobenzene is any of three isomeric chlorinated derivatives with the molecular formula C6H2Cl4. They differ by the positions of the chlorine atoms around the ring. Tetrachlorobenzenes are colorless crystalline compounds.












